All published articles of this journal are available on ScienceDirect.
Impact of Intraoral Use and Cleaning Methods on the Performance of Stainless-steel Orthodontic Archwires: A Clinical Ex-vivo Study
Abstract
Aim
This study aimed to evaluate the effects of intraoral use and two cleaning methods on debris accumulation, surface roughness (SR), and friction force of two brands of stainless steel (SS) archwires, 3B Ortho and American Orthodontics (AO), after 8 weeks of intraoral exposure.
Material and Methods
The sample consisted of 288 SS orthodontic archwire sections (0.019” × 0.025”), equally divided between AO and 3B brands. It included an as-received control group (n = 72) and a retrieved group from 54 patients after 8 weeks of use (n = 216). The retrieved wires were further categorized into cleaning method groups: non-cleaning, alcohol-soaked gauze (A-gauze), and acetone-soaked gauze (Ac-gauze). Debris was evaluated using scanning electron microscopy, SR was measured with a Talysurf-i60 profilometer, and frictional forces were assessed using a universal testing machine. Two-way ANOVA was used to compare the effects of different cleaning methods on SS archwires and between the two brands of SS archwires. A significance level of p < 0.05 was used.
Results
The retrieved group had the highest median debris score of 4, whereas the as-received group recorded a score of 0. Alcohol-soaked and acetone-soaked gauzes yielded decreased debris scores of 1. For SR, the retrieved group had a mean of 0.533 compared with the A-gauze group (0.047). In terms of frictional forces, the retrieved group had an average force of 4.74, and the A-gauze and Ac-gauze groups averaged 2.58 and 3.8, respectively. No significant differences in debris levels were found between brands, but the 3B brand had significantly higher SR (mean = 0.24) than the AO brand (mean = 0.17, p < 0.001).
Discussion
Intraoral aging significantly increased debris, SR, and friction in stainless steel archwires. A-gauze and Ac-gauze cleaning methods effectively reduced these parameters, with A-gauze demonstrating superior efficacy, especially for the 3B brand. Despite no brand differences in debris or friction, routine chairside cleaning is critical to maintaining archwire performance. Ac-gauze is a viable alternative for debris removal when alcohol is unavailable.
Conclusion
The retrieved SS archwires exhibited increased debris, SR, and friction after 8 weeks of intraoral use. Both A-gauze and Ac-gauze cleaning methods effectively reduced debris and friction, with A-gauze showing greater overall efficacy, particularly for the 3B brand, which demonstrated higher SR compared to AO.
1. INTRODUCTION
The prolonged presence of fixed orthodontic appliances in the oral cavity exposes stainless steel (SS) archwires to biofilm formation and calcified debris accumulation, which adversely affect their surface roughness (SR) and frictional properties, ultimately impacting treatment efficacy [1, 2]. Increased SR, resulting from biofilm and debris, elevates friction at the bracket-wire interface, impeding sliding mechanics and reducing the efficiency of tooth movement [3-5]. This heightened frictional force can hinder tooth movement and compromise treatment progress [5].
The friction between the wire and bracket during orthodontic treatment is a crucial factor that can significantly impact the efficacy of tooth movement [6]. In orthodontic mechanotherapy, the effective force is the force that surpasses the friction at the bracket-wire interface, allowing tooth movement to occur. However, if the frictional forces exceed the applied force, the system’s efficiency can be compromised, potentially prolonging treatment time or affecting results due to insufficient tooth movement or loss of anchorage [7]. Studies have shown that friction between the bracket and wire can lead to a loss of the applied orthodontic force ranging from 12 to 60% [8].
Numerous in vitro studies have explored friction in various combinations of wires and brackets, considering differences in alloy type, bracket type, and ligation methods [7-10]. The selection of appropriate materials for the bracket-archwire assembly is crucial for facilitating effective tooth movement via sliding mechanics because it directly influences the coefficient of friction and SR [11]. Rectangular SS wires are especially advantageous for mechanical sliding due to their reduced coefficient of friction and smooth surface [12].
However, the 2-month presence of SS archwires in the oral environment shows considerable debris accumulation, as revealed by scanning electron microscopy (SEM) [4, 13]. This debris can damage the protective surface layer of the SS and initiate a corrosion process [14, 15]. Such degradation promotes further debris accumulation on archwires, with a significant correlation observed between debris presence and increased friction [4, 13]. Other studies reported that cleaning the orthodontic archwire effectively reduces the elevated levels of frictional resistance between the archwire and bracket surfaces during sliding mechanics [3, 16, 17].
Marques et al. (2010) recommended cleaning archwires at each visit to prevent debris accumulation and preserve the intrinsic properties of stainless steel archwires [13]. Various archwire cleaning methods have been investigated, such as sodium bicarbonate jet, alcohol-soaked gauze, ultrasonic cleaner, and steel wool sponge with alcohol-soaked gauze cleaning, which was the most efficient in debris removal without causing damage to the archwire surface [3, 16, 17]. Other studies investigated the use of acetone (propanone) for cleaning endodontic files to ensure proper cleaning and disinfection against clogged materials and bacterial pathogens [18]. No previous studies attempted to evaluate its use for cleaning orthodontic archwires between appointments.
In Yemen, the prevailing economic conditions have resulted in 3B Ortho and American Orthodontics being the most available and widely used brands of SS archwires. In addition, these two brands offered the required properties during the treatment follow-up time. Given the lack of published studies on the use of acetone as a cleaning method for preformed orthodontic SS archwires, this study was designed to investigate the effects of alcohol-soaked gauze (A-gauze) and acetone-soaked gauze (Ac-gauze) on debris accumulation, SR, and friction force in SS archwires after 8 weeks of intraoral use. The effects of these cleaning methods on two brands (3B and AO) of 0.019″ × 0.025″ preformed orthodontic SS archwires before and after the 8-week intraoral exposure were examined.
2. MATERIALS AND METHODS
2.1. Study Design and Ethical Approval
This clinical ex vivo study was approved by the Ethics Committee of the Faculty of Medicine and Health Sciences at USTY (MECA No.: EAC/UST170). Consent was obtained by asking subjects to sign a form that explained the nature and purpose of the investigation.
2.2. Sample Size Calculation
The sample size was determined using OpenEpi statistical calculation software, with a confidence level of 95%, a power of 80%, and an α level of 0.05. On the basis of previous data [16], a mean difference of 1.02 N was reported between the control (T0) and retrieved (T1) groups, with standard deviations of 0.43 and 0.96 N, respectively. The minimum sample size was 12 archwire sections per group, resulting in a total of 288 archwire samples.
2.3. Inclusion Criteria
Two brands of SS archwires were used: AO American Orthodontics (American Orthodontics Corporation, Sheboygan, Wisconsin, USA) and 3B Ortho (Hangzhou Xingchen 3B Dental Instrument & Material Co. Ltd., Hangzhou, China). Both archwires were 0.019” × 0.025” in size, had a preformed shape, and were FDA-certified. They did not undergo any bending or notching before insertion. The archwires were collected from patients who had fixed appliances and undergone first premolar extractions as part of their treatment protocol while maintaining good oral hygiene and on the verge of completing their first stage (leveling and alignment) of treatment.
2.4. Exclusion Criteria
Exclusion criteria included patients with poor oral hygiene, archwires that had been bent, notched, or otherwise modified before insertion, and archwires retrieved from patients who did not complete the 8-week intraoral exposure period or had incomplete clinical records. Non-FDA-certified archwires or those with dimensions other than 0.019” × 0.025”, as well as patients undergoing orthodontic treatment protocols that did not include first premolar extractions, were also excluded.
2.5. Patient Grouping and Archwire Bonding
This study included two equal groups of preformed SS archwires: group A consisted of archwires in their as-received state, whereas Group B comprised archwires retrieved from patients undergoing orthodontic treatment.
Bonding involved the placement of fixed appliances with a 0.022 × 0.028-inch slot MBT bracket system (SIA, Italy) with wire dimensions of 0.019″ × 0.025″ inch. Each archwire was inserted and ligated with elastomeric modules, except for canines where 0.010 SS ligature wire was used. A closed elastic chain was placed between the first molar and canine, exerting a force of 150 g, according to Wahab et al. (2015), as measured by the force gauge provided [19]. After 8 weeks of intraoral exposure, the retrieved archwires were cut into 216 hemi-arch samples, comprising 108 segments from each brand. The segmented retrieved SS archwire samples were distributed randomly into three groups based on the cleaning method, using Research Randomizer software (version 4.0, Lancaster, PA, USA).
The non-cleaning test group consisted of 36 segments from each brand, with no cleaning applied. For the cleaning test groups, 72 segments from each brand were allocated: one group received cleaning with A-gauze, and the other group was cleaned with Ac-gauze. Fig. (1) shows the flowchart of this study.
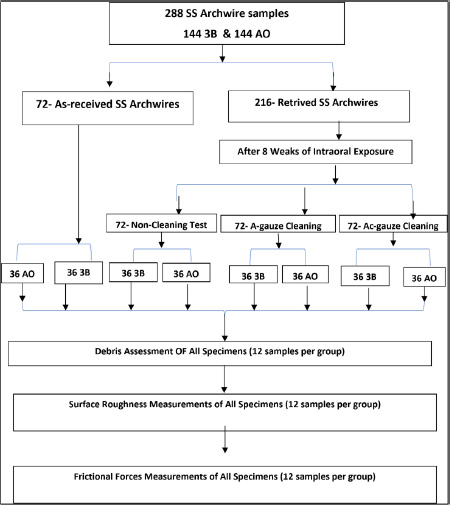
Flowchart of this study.
The cleaning process for the A-gauze group involved clamping the wire segments with mosquito forceps, soaking gauze in 77% alcohol, and rubbing the wire for 20 s, as shown in Fig. (2). For the Ac-gauze group, the process was similar, but 90% acetone was used instead. Both concentrations effectively dissolved organic and calcified debris [20]. All cleaned segments were then labeled and packaged for evaluation.
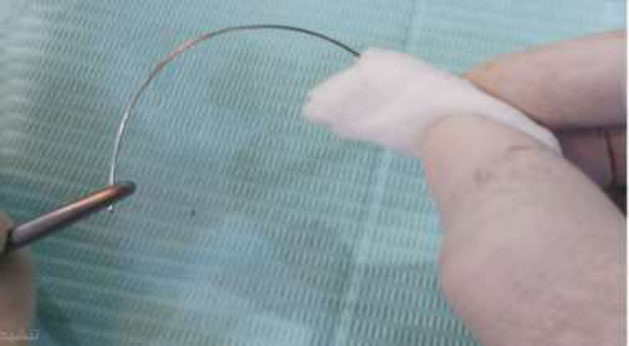
Alcohol-soaked gauze cleaning method (A-gauze).
The outcomes were categorized into three measurement types: debris measurement, SR measurement, and frictional force measurement. Each group was organized into specific folders for analysis, and all evaluations were conducted within 48 h of archwire retrieval. The debris amount, SR, and frictional forces of SS archwire samples were assessed in the control and test groups by the same operator to maintain consistency, reducing variability and potential bias in measurement.
Each 10 mm segment was cut from the distal aspect of the canine bracket and fixed with double-sided tape on a 22 mm × 22 mm glass slide. The central area of each sample was marked to standardize the debris assessments.
Debris was evaluated by SEM using a Quanta 250 FEG (Drive Hillsboro, Oregon, USA) at 2000× magnification, and images were obtained with xT microscope Control Software (Fig. 3). From these images, a modified debris index was used for evaluation following the criteria of Marques et al. (2010) [13], as shown in Table 1.
| Score | Criteria |
|---|---|
| 0 | Total absence of debris |
| 1 | Debris covering up to 25% |
| 2 | Debris covering between 25–50% |
| 3 | Debris covering between 50–75%. |
| 4 | Debris covering over 75% |
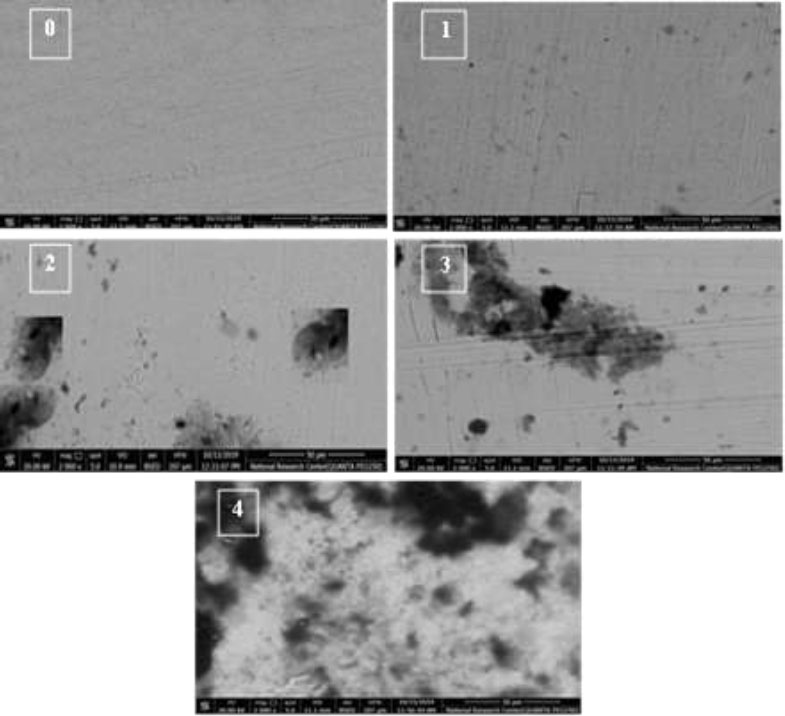
SEM images show the amount of debris at 2000× magnification.
2.6. Evaluation of SR
The test sample and control group wires were examined for SR by the same operator using a surface profilometer. A 20 mm piece of archwire was cut from the distal canine bracket for standardization. The samples were placed in a Talysurf-i60 contact stylus profilometer (Taylor Hobson, England, S. No. 4932, U = ± 0.3 nm). The machine automatically determined the mean roughness from 12 profilometric scans per sample. Arithmetic mean roughness was measured in micrometers in accordance with ISO 3274:1996, with a cut-off of 5 × 0.8 mm and a scanning speed of 0.5 mm/s. Data were digitized using Metrology 4.0 Smart Software (Taylor Hobson, Ltd.). Uncertainty evaluation was carried out in accordance with JCGM 100:2008 as follows: U = ± 0.100 µm (where U is the expanded uncertainty using a coverage factor K = 2), providing a level of confidence of approximately 95%. All measuring equipment was traceable to roughness standards calibrated at VTT MIKES, Finland (Certificate No. M-19L090). Measurements were conducted at a controlled temperature of 20 ± 1 °C.

Test setup with acrylic block model in instron universal testing machine.
2.7. Measurements of Frictional Forces
A metal MBT bracket (Shinye, China) with a 0.022 × 0.028-inch slot, 0° angulation, and 0° torque was used to test the frictional forces under dry conditions. The bracket was bonded to the upper right second premolar by using a light-cure composite (SIA, Italy), and the tooth was encased in an acrylic block (30 mm × 20 mm × 15 mm). The wire was tied to the brackets by using elastic ligature (0.12-in DTC, China). Samples were mounted on a universal testing machine (Model 3345; Instron Industrial Products, Norwood, MA, USA) with a load cell of 5 kN, and data were recorded using computer software (Instron Bluehill Lite Software). The acrylic block was secured to the machine’s lower compartment, and the wire was attached to the upper compartment and extended at a crosshead speed of 1 mm/min up to a distance of 5 mm, as shown in Fig. (4). The kinetic frictional force was measured in Newtons (N) as the mean force exerted from the initiation of movement to the completion of the test. The maximum static friction force was recorded at the onset of movement, while the kinetic frictional force was calculated as the average force during displacement from 1 mm to 5 mm, using Bluehill® Central lab management software (Instron®).
All clinical assessments and measurements were carried out by the principal investigator.
In the course of this clinical ex vivo study, the principal investigator (A.M.A) executed the bonding of archwires for the cleaning and non-cleaning groups, employing clinical steps alongside non-cleaning, A-gauze, and Ac-gauze cleaning tests for the materials under examination. The laboratory parameters, such as debris assessments, SR by SEM, and frictional force measurements, were conducted with the help of the mechanical dental materials manager.
2.8. Intra-examiner Reliability
Intra-examiner calibration was performed, which involved the measurement of clinically assessed parameters for 105 patients at 10-day intervals. The samples and their values were incorporated into the total sample of the current study. The results showed a strong correlation, with a coefficient of 0.948, indicating high agreement.
2.9. Statistical Analysis
The debris index scores showed a non-normal distribution (nonparametric), whereas the SR and frictional force data showed a normal distribution (parametric distribution). Therefore, the descriptive statistics were presented as mean and standard deviation (±SD) for parametric data, and nonparametric data were presented as median and interquartile range. Data were entered and analyzed using SPSS software (version 23.0; IBM Corp., Armonk, NY, USA). Two-way ANOVA was used to evaluate the effects of different cleaning methods on SS archwires and compare the two brands of SS archwires. p > 0.05 was set at the significant level.
3. RESULTS
The descriptive analysis of the sample is presented in Table 2. For the 3B and AO brands, the as-received group had a median score of 0 (IQR = 0), but the retrieved samples that were maintained for 8 weeks in the oral environment showed a significant increase in the debris. The calculated median score for the two retrieved groups was 4 at 2000× magnification. By contrast, the value decreased and had a median score of 1 (IQR = 0) after cleaning with A-gauze and Ac-gauze.
In terms of SR (Ra), the 3B brand had a mean roughness of 0.059 for the as-received samples, which increased to 0.503 after retrieval (no cleaning) and then decreased to 0.053 and 0.346 for the A-gauze and Ac-gauze groups, respectively. The AO brand showed a similar trend, with a mean roughness of 0.037 for the as-received samples, increasing to 0.563 after retrieval and decreasing to 0.041 and 0.039 for the A-gauze and Ac-gauze groups, respectively.
| Group | Brand | Debris | Surface Roughness | Friction | |||
|---|---|---|---|---|---|---|---|
| Median | IQR | Mean | SD | Mean | SD | ||
| As-Received | 3B | 0 | 0 | 0.059 | 0.001 | 2.983 | 0.413 |
| AO | 0 | 0 | 0.037 | 0.001 | 3.525 | 0.286 | |
| Non-cleaning | 3B | 4 | 0 | 0.503 | 0.001 | 5.042 | 0.601 |
| AO | 4 | 0 | 0.563 | 0.001 | 4.442 | 0.425 | |
| A-gauze | 3B | 1 | 0 | 0.053 | 0.001 | 2.475 | 0.160 |
| AO | 1 | 0 | 0.041 | 0.001 | 2.675 | 0.129 | |
| Ac-gauze | 3B | 1 | 0 | 0.346 | 0.001 | 3.733 | 0.328 |
| AO | 1 | 0 | 0.039 | 0.001 | 3.875 | 0.328 | |
| Total | - | 1.5 | 0 | 0.205 | 0.214 | 3.590 | 0.901 |
| Variable | Debris | SR | Friction | |||
|---|---|---|---|---|---|---|
| Median | IQR | Mean | SD | Mean | SD | |
| As-received | 0 | 0 | 0.048 | 0.011 | 3.25 | 0.44 |
| Non-cleaning | 4 | 0 | 0.533 | 0.031 | 4.74 | 0.59 |
| A-gauze | 1 | 0 | 0.047 | 0.006 | 2.58 | 0.18 |
| Ac-gauze | 1 | 0 | 0.192 | 0.157 | 3.8 | 0.39 |
| p-value | <0.001* | <0.001* | <0.001* | |||
| Variable | 3B | AO | p-value | ||
|---|---|---|---|---|---|
| M/Med | SD/IQR | M/Med | SD/IQR | ||
| Debris | 1.5 | 0 | 1.5 | 0 | >1.000 |
| SR (Ra) | 0.24 | 0.194 | 0.17 | 0.229 | <0.001* |
| Friction (F) | 3.56 | 1.06 | 3.63 | 0.71 | 0.409 |
For friction, the 3B brand exhibited a mean friction force of 2.983 N for the as-received samples, increasing to 5.042 N after retrieval and then decreasing to 2.475 and 3.733 N for the A-gauze and Ac-gauze groups, respectively. The AO brand had a mean friction force of 3.525 N for the as-received samples, increasing to 4.442 N after retrieval and decreasing to 2.675 and 3.875 N for the A-gauze and Ac-gauze groups, respectively.
Two-way ANOVA analysis of SS archwires revealed significant differences in debris, SR, and frictional forces across various groups. The retrieved group had the highest median debris, followed by the A-gauze and Ac-gauze groups, whereas the as-received group had the lowest median debris (Table 3). For SR, the retrieved group exhibited the highest mean at 0.533, and the Ac-gauze group had a mean of 0.192. The as-received and A-gauze groups showed the lowest SR values. In terms of frictional forces, the retrieved group had the highest mean of 4.74, followed by the Ac-gauze group at 3.8, the as-received group at 3.25, and the A-gauze group at 2.58, indicating significant differences among the cleaning methods.
Fig. (5) graphically shows the comparison of the effects of cleaning methods on SS archwire samples. The differences in mean scores between the AO and 3B brands of SS archwire samples are presented in Table 4. The findings showed no significant differences in debris levels between the two brands, with p > 1.000. Regarding SR, the 3B brand had a significantly higher mean (0.24) than the AO brand (0.17), with p < 0.001. For frictional forces, no significant difference was observed between the brands, with p < 0.409.
4. DISCUSSION
Rectangular SS archwires are considered the most popular archwires used during the space closure stage of orthodontic treatment due to their low friction, SR, elastic modulus, high formability, and biocompatibility. SS archwires are exposed to the intraoral environment for varying durations that can reach several months. The effects of intraoral aging include the accumulation of debris and an increase in SR and frictional forces [13]. Some researchers suggested cleaning the archwire at recall visits to restore
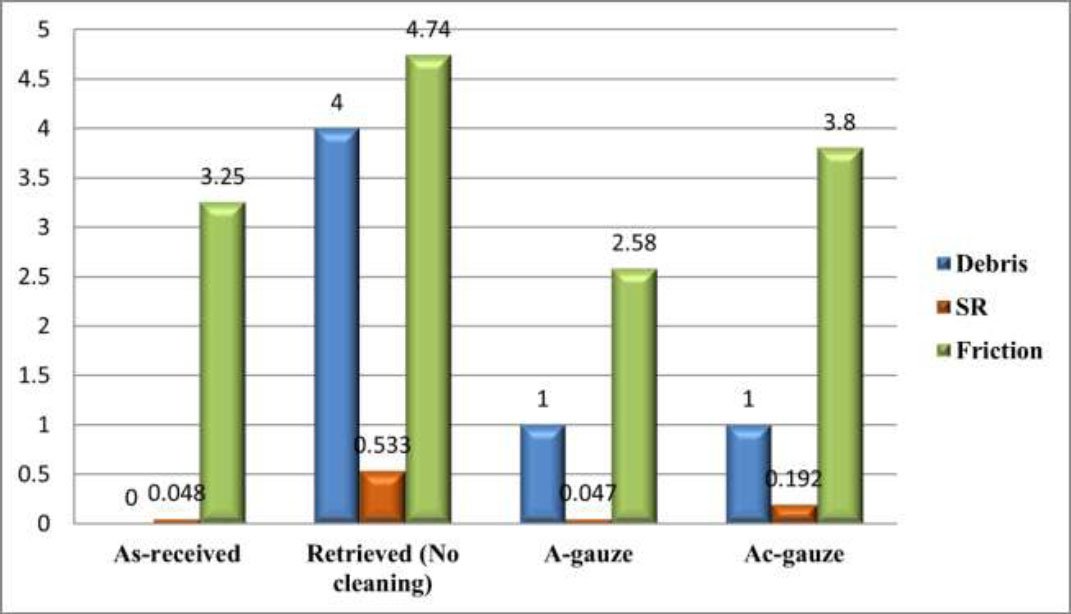
Comparison of the effects of cleaning methods on SS archwires regardless of archwire brands.
its surface properties for performance optimization [13, 16]. However, few studies evaluated the efficiency of cleaning methods on the levels of debris, SR, and friction of SS orthodontic archwires [3, 16, 17]. Thus, this study aimed to investigate the effects of two cleaning methods on two brands of SS archwires after 8 weeks of intraoral use.
Intraoral biofilm maturation and calcification typically progress over weeks. An 8-week period provides sufficient time for clinically meaningful debris accumulation and SR changes, as shown by Marques et al. [13]. Short durations (e.g., 4 weeks) may not yield measurable changes, whereas long periods (e.g., 12 weeks) risk introducing factors such as wire fatigue or patient non-compliance. Previous studies used similar timeframes (6–12 weeks), making 8 weeks a valid midpoint for detecting significant changes in debris, SR, and friction [4, 13].
The results revealed that the as-received group had no visible debris, but the 3B and AO groups showed a median number of debris particles of 4 after being retrieved from the oral environment. However, when the archwires were cleaned with A-gauze or Ac-gauze for 20 s, the median number of debris particles significantly decreased to 1, indicating that debris covered less than 25% of the SEM images for the cleaned groups compared with over 75% for the non-cleaned group (p < 0.001). This finding was consistent with those of previous studies by Normando et al. (2013) and Nikhil et al. (2020), who also demonstrated the effectiveness of cleaning methods in reducing debris accumulation on SS archwires [3, 16]. However, our results differed from those of Mattiello et al. (2018), who used U-sonic for 6 min and sodium bicarbonate jet for 30 s, thereby suggesting that these methods may not be as effective in controlling debris accumulation [17]. This difference was ascribed to the variation in cleaning methods and evaluation periods across studies.
SR is an important property of orthodontic archwires because it affects corrosion behavior, biocompatibility, appearance, hygiene, and friction during tooth movement [12]. The present study revealed that all the as-received SS archwire samples exhibited the lowest mean of SR, but after 8 weeks of intraoral use, the retrieved SS archwires showed a significant increase in the mean SR (Table 1), similar to the results of previous studies [3-5, 13, 17]. However, the retrieved SS archwires cleaned with either A-gauze or Ac-gauze for 20 s showed significantly reduced SR, with average Ra values of 0.047 and 0.192 μm, respectively, compared with an average Ra of 0.533 μm for the non-cleaned group (p < 0.001). This finding indicated that cleaning with A-gauze or Ac-gauze effectively reduced SR in non-cleaned SS archwires, which was aligned with the findings of Normando et al. (2013) and similar to those of Mattiello et al. (2018), who used SWS for 30 s [16, 17]. However, the results differed from those of Mattiello et al. (2018) when using A-gauze for 20 s, U-sonic for 6 min, and sodium bicarbonate jet for 30 s. The results also differed from those of Talic et al. (2017), who used a sodium bicarbonate jet for 1 minute and found that it failed to control SR and may even contribute to its increase [17, 20].
All the retrieved SS archwires cleaned with A-gauze or Ac-gauze for 20 s showed significantly reduced frictional forces, averaging 2.58 and 3.8 N, respectively, compared with 4.74 N for the non-cleaned group (p < 0.001). Thus, cleaning with A-gauze or Ac-gauze for 20 s was effective in reducing frictional forces in non-cleaned SS archwires, consistent with the findings of Normando et al. (2013) and Nikhil et al. (2020) [3, 18]. However, the results differed from those of Mattiello et al. (2018), who found that cleaning methods like SWS, A-gauze, U-sonic, and SB-jet do not alter static friction [17]. This inconsistency suggests that frictional forces in stainless steel archwires may not be significantly altered after a short duration of intraoral use.
The comparison of the effects of A-gauze and Ac-gauze cleaning methods showed that Ac-gauze was less effective than A-gauze at reducing the SR of SS archwires, with Ra values of 0.192 and 0.047 μm, respectively, compared with 0.533 μm for no cleaning. For the 3B brand, Ac-gauze showed lower effectiveness (Ra = 0.346 μm) than A-gauze (Ra = 0.053 μm) compared with no cleaning (0.503 μm). By contrast, the effectiveness of both methods was similar for the AO brand, with Ra values of 0.039 μm for Ac-gauze and 0.041 μm for A-gauze, reduced from 0.563 μm. Ac-gauze cleaning was less effective than A-gauze cleaning in reducing the frictional forces of SS archwires, with mean values of 3.8 and 2.58 N, respectively, compared with 4.74 N for no cleaning. Nonetheless, Ac-gauze can still be clinically applied as an effective method for decreasing frictional forces in SS archwires, as shown in Fig. (5).
The mechanical properties of orthodontic archwires can vary significantly between different manufacturers, even when made from similar materials and dimensions [6]. The present study found no significant difference in debris scores between the 3B and AO archwires, consistent with previous studies on 3M Unitek SS archwires [3, 4, 13, 16, 17, 21]. However, the 3B archwires had a significantly higher SR than the AO archwires (0.059 versus 0.037 μm, p < 0.001). This result was supported by the findings of Facchini et al. (2017), who compared the SR of seven brands of SS archwires before clinical use and identified differences in the SR orthodontic archwires among the commercial brands studied [22]. Although no significant differences in frictional forces were observed between brands in this study, the elevated SR of 3B wires may increase biofilm retention and corrosion risk, potentially compromising long-term performance. Clinically, rough surfaces may impede sliding mechanics; however, A-gauze effectively reduced 3B’s SR to near baseline levels, mitigating this risk. AO’s reduced SR may offer minor benefits in friction-sensitive scenarios, such as space closure. Brand selection should align with clinical needs, balancing SR characteristics with maintenance protocols to ensure efficient treatment and minimize biomechanical inefficiencies.
The findings of this study have significant clinical implications for orthodontic practice as it demonstrates that 20 s of A-gauze cleaning at every appointment reduces debris, SR, and friction in archwires, supporting routine chairside cleaning during adjustments to improve treatment efficiency. This protocol minimizes friction in tooth movement, especially during sliding mechanics (e.g., space closure). Although acetone is less effective than alcohol in reducing SR due to its rapid evaporation, which limits cleaning time, it remains useful for removing debris, especially calcified deposits, or when alcohol is unavailable. Acetone’s strong solvent properties effectively dissolve organic debris and biofilm on archwires. Although it has been implemented in other dental applications, its use for archwire cleaning is novel, offering a viable method to restore performance and optimize orthodontic outcomes.
SS’s protective chromium oxide layer is generally resistant to organic solvents like acetone and alcohol. However, acetone’s strong solvency (a polar aprotic solvent) may disrupt adsorbed organic debris aggressively and transiently interact with surface contaminants, leaving micro-abrasions if mechanical rubbing is involved. Alcohol is less aggressive than acetone and offers a balanced cleaning effect, effectively dissolving organic biofilm without damaging the oxide layer [18]. The study’s methodology involved gauze rubbing, which, when combined with acetone’s rapid evaporation, might limit contact time, reducing its efficacy in thoroughly dissolving calcified deposits or biofilm residues compared with alcohol.
5. STUDY LIMITATION
A limitation of this study is the exclusion of saliva's lubricating effect during frictional force testing, which was conducted under dry laboratory conditions. Saliva serves as a natural lubricant, forming a protein-rich biofilm on archwires and brackets that can influence friction dynamics [4]. The dry testing environment likely overestimates frictional forces compared with intraoral conditions, where lubrication may reduce the differences between cleaned and non-cleaned groups. Although this study examined the effects of two cleaning methods on two SS archwire brands regarding three aspects of intraoral aging, it did not analyze changes in the structural characteristics of the archwire surface. Future studies should consider environmental factors such as diet, salivary composition, and patient-specific oral hygiene behaviors to evaluate friction and SR under hydrated conditions. Additionally, investigating extended time intervals of cleaning could offer further insights into factors influencing archwire mechanical properties and degradation in the intraoral environment and alternative cleaning methods.
CONCLUSION
Within the limitations of this study, and after 8 weeks of simulating clinical implications, SS archwires from the 3B and AO brands showed significant increases in debris accumulation, SR, and frictional forces. Cleaning with A-gauze or Ac-gauze for 20 s effectively reduced these parameters, with A-gauze demonstrating superior efficacy, especially for the 3B brand. Although no brand differences were noted in debris or friction, clinicians should prioritize routine cleaning, especially with A-gauze during friction-sensitive phases like space closure, to maintain performance. Ac-gauze is an effective alternative for debris removal when alcohol is unavailable, helping to maintain archwire functionality and enhance treatment efficacy.
AUTHORS’ CONTRIBUTIONS
A.M.A.R.: Data collection; M.A.A-L.: Data analysis or interpretation; R.A.R.I.: Methodology; T.A.B.: Writing - reviewing and editing; H.S.A.A-Z.: Investigation; M.M.A-M.: Writing - original draft preparation.
LIST OF ABBREVIATIONS
| SS | = Stainless Steel |
| SR | = Surface Roughness |
| SEM | = Scanning Electron Microscopy |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This clinical ex vivo study was approved by the Ethics Committee of the Faculty of Medicine and Health Sciences at USTY, Yemen (MECA No.: EAC/UST170).
HUMAN AND ANIMAL RIGHTS
All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committees and with the 1975 Declaration of Helsinki, as revised in 2013.
AVAILABILITY OF DATA AND MATERIALS
The data and supportive information are available within the article.
CONFLICT OF INTEREST
Mohammed M. Al Moaleem is the Editorial Advisory Board member of The Open Dentistry Journal.
ACKNOWLEDGEMENTS
Declared none.


