All published articles of this journal are available on ScienceDirect.
Penetration of Universal Adhesive System after Smear Layer Removal on Dentin Using Tamarindus indica Solution
Abstract
Background
The smear layer causes a weak bond to dentin; therefore, removal of the smear layer with ethylenediamine tetraacetic acid (EDTA) solution was recommended. EDTA can cause erosion and changes in dentin’s microhardness. Hence, natural products that are biocompatible and have fewer side effects are being developed, one of which is Tamarindus indica. Tamarindus indica solution contains organic acids (citric acid, acetic acid, and maleic acid), therefore, it can dissolve minerals (demineralization), remove the smear layer, and act as a chelating agent.
Objective
To compare the penetration of the universal adhesive system after smear layer removal on dentin using Tamarindus indica 2,5%, 5%, 10%, and 17% EDTA solution.
Methods
Premolars (n=24) had their enamel removed, exposing the dentin. Four groups of smear layer removal agents, Tamarindus indica 2,5%, 5%, 10%, and 17% EDTA were applied to dentin. A universal adhesive system was then applied, restored with composite resin, and incubated at 37° for 24 hours. Penetration of the universal adhesive system was observed using a Scanning Electron Microscope.
Results
There was a significant difference in the penetration of universal adhesive after smear layer removal between Tamarindus indica 10% group and Tamarindus indica 2.5%, 5%, and EDTA 17% group (p <0.05). The longest resin tag penetration was found in Tamarindus indica 10% compared to Tamarindus indica 2,5%, 5%, and EDTA 17%.
Conclusion
10% Tamarindus indica solution was effective in the removal of the smear layer and resulted in longer penetration of resin tags compared to 2.5% Tamarindus indica, 5% Tamarindus indica, and 17% EDTA.
1. INTRODUCTION
Tooth preparation and caries excavation using rotary and manual instruments will produce a smear layer [1, 2]. The smear layer is a zone of debris on the tooth surface, which consists of crushed hydroxyapatite and collagen denatured by friction and heat during tooth preparation [3]. It is still debatable whether the smear layer should be present underneath the restoration. The smear layer acts as a natural barrier and reduces dentin permeability up to 86% by sealing the tubules and limiting the penetration of bacterial toxins, however, the smear layer is a porous layer that only forms a weak attachment to the underlying dentin, which will disintegrate over time and cause microleakage [2].
Removal of the smear layer before bonding or the use of bonding material that can penetrate and incorporate it was done to overcome the weak bond strength of the smear layer.
The first adhesive systems were based on smear layer removal (etch and rinse). Phosphoric acid etching can cause dentin permeability up to 90% and induce postoperative tooth sensitivity, causing aggressive demineralization, thereby exposing more dentin collagen fibrils and degrading the exposed collagen over time [1, 3]. Self-Etch (SE) adhesive systems do not require etching and rinsing steps, resulting in less post-operative sensitivity [4]. In self-etch adhesive systems, acid monomers can dissolve the smear layer or incorporate it into the bonding interface [5]. Dentin demineralization and resin infiltration that occur simultaneously can prevent the formation of unprotected collagen tissue; however, when it penetrates into dentin, there can be a buffering effect of acid monomers by the smear layer and the mineral content in dentin due to the weak acid content of the self-etch adhesive system [3, 6]. The latest generation of adhesive systems are universal or multi-mode adhesive systems that can be used as self-etch, etch and rinse, or selective enamel etching adhesive systems [7]. These adhesive systems are mostly classified as light and very light SE and have functional monomers, 10-Methacryloyloxydecyl Phosphate (MDP) or Glycerol Phosphate Dimethacrylate (GPDM) that has a strong bond to hydroxyapatite [8].
Lack of ability to penetrate through a thick smear layer can cause reduced bond strength and failure of the restoration. To increase infiltration into the dentin, removal of the smear layer on dentin is recommended to facilitate diffusion of the resin through the mineralized dentin matrix [6]. The application of acidic or chelating solutions can achieve complete or partial removal of the smear layer. Ethylenediamine tetraacetic acid (EDTA) is a polyaminocarboxylic acid that can react with calcium ions in dentin to form calcium chelate, and can lightly demineralize dentin [9, 10]. Conditioning dentin with EDTA can increase bond durability due to shallow demineralization of hydroxyapatite, especially in deeper parts of dentin [5]. Studies by Kim showed that the use of EDTA as a substitute for 37% phosphoric acid minimized the layer of collagen fibrils that were not completely infiltrated by resin, providing good resin-dentin bond durability [11]. Ethylenediamine tetraacetic acid (EDTA), however can cause allergic reactions, erosion of dentin, and changes in microhardness. Hence, natural products have been developed because of their biocompatibility and fewer side effects compared to synthetic products [12].
One of the natural smear layer removal agents that is currently being developed is Tamarindus indica (tamarind) fruit extract. Tamarindus indica contains several organic acids, including citric acid, acetic acid, and maleic acid, that are similar to EDTA, so they can be used to remove the smear layers. The acid content of Tamarindus indica can dissolve minerals (demineralization) and function as a chelating agent [13, 14]. 5% Tamarindus indica solution has greater smear layer cleaning ability and lower toxicity effects than 3% H2O2. Tamarindus indica is a potential source of antimicrobials and has antibacterial activity against Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, and Streptococcus mutans [15]. Tamarindus indica solution has been widely used in previous research as an irrigation solution to remove the smear layer in the root canal. This research uses Tamarindus indica solution to remove the smear layer on dentin.
2. MATERIALS AND METHODS
This in vitro experimental study obtained ethical approval from the Dental Research Committee (ethical clearance number: 11/Ethical Approval/FKGUI/III/2023). Tamarindus indica (tamarind) fruits in this study were taken from Babakan village, Darmaga, Bogor. Tamarindus indica (tamarind) extract was made into a paste and then extracted at a concentration of 2.5%, 5%, and 10%. A total of 24 extracted sound human premolars without caries, cracks, or restorations were collected as research samples. The teeth were cleaned and then placed in a Phosphate-Buffered Saline (PBS) solution.
Preparation of the samples (a) The teeth were cut at the occlusal, buccal, lingual, mesial, and distal, removing the enamel and exposing the dentin surface, (b) Composite resin was placed on the teeth, and samples were cut parallel to the tooth axis with a chisel to produce samples that were free from the smear layers.
RK: composite resin; b: universal adhesive system; T: resin tag.
The teeth were cut at the occlusal, buccal, lingual, mesial, and distal using a diamond disc bur to remove the enamel and expose the dentin surface (Fig. 1a). The crowns were separated from the root at the Cemento-Enamel Junction (CEJ). Samples were divided into 4 groups: 2.5%, 5%, 10% Tamarindus indica, and 17% EDTA (MD Cleanser, Meta Biomed, South Korea). Smear layer removal agents were applied for 60 seconds using a microbrush and then rinsed with water for 10 seconds. After the application of the smear layer removal agents, the bonding procedures were performed with a universal adhesive system (OptiBond Extra Universal, Kerr, USA). OptiBond Extra Universal Primer was applied to the teeth using a micro brush in rubbing motion for 20 seconds and then was air sprayed for 5 seconds. OptiBond Extra Universal Adhesive was then applied in rubbing motion for 15 seconds, spread evenly with air spray for 5 seconds, and light-cured for 10 seconds. Composite resin (Filtek Z250, 3M ESPE, USA) was placed into the molds (diameter 2 mm, height 2 mm) on the teeth, and then each layer was light-cured for 20 seconds. The samples were then incubated at 37°C for 24 hours. Teeth were then cut parallel to the tooth axis with a chisel to produce samples that were free from the smear layers (Fig. 1b).
The samples were mounted on aluminum stubs and coated with gold using a sputtering device. Samples were examined using a scanning electron microscope (Hitachi, SU 3500, Japan) operating at 15 kV with 1000x, 2500x, 5000x, and 8000x magnifications. Data on the penetration depth of the adhesive system were analyzed using SPSS software (version 26.0, IBM Corp., New York, USA).
3. RESULTS
The morphology features of the universal adhesive system after smear layer removal on dentin can be observed in Fig. (2). It can be seen that each group has a good adaptation of composite resin, adhesive system, and dentin. A hybrid layer can be found at the interface between resin and dentin, which was indicated by a dense layer. Resin tags were seen in each group by the penetration of the adhesive system into the dentin tubules.
The longest resin tag penetration was found in the 10% Tamarindus indica group (Fig. 2c) when compared to the 2.5% Tamarindus indica, 5% Tamarindus indica, and 17% EDTA groups (Fig. 2a, b, d). The shortest resin tag penetration can be found in the 17% EDTA group (Fig. 2d).
The thickness of the adhesive layer can be seen between the composite resin and dentin (Fig. 2). Scanning electron microscope images showed that 2.5% Tamarindus indica (Fig. 2a) had the thinnest adhesive layer compared to the other groups. The adhesive layer at the resin-dentin interface appeared slightly thicker in the 5% Tamarindus indica group (Fig. 2b), and the thickest adhesive layers were found in the 10% Tamarindus indica and 17% EDTA groups.
The data obtained were then analyzed using SPSS 26 software. The normality test was carried out, and a normal distribution was obtained. Thus, a one-way ANOVA test was carried out. In the homogeneity test, the data were homogeneous, so the Bonferroni post hoc test was performed. Table 1 shows that the 10% Tamarindus indica group had the longest universal adhesive penetration after smear layer removal, 17 (SD 0.99) µm, while the 17% EDTA group had the shortest universal adhesive penetration value after smear layer removal, 12.83 (SD 1.83) µm. One-way ANOVA test showed a value of 0.00 (p<0.05), indicating that there was a significant difference in the penetration of the universal adhesive system after the removal of the smear layer in all groups (Table 1).
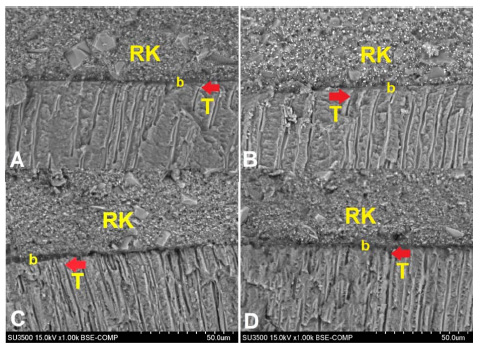
Scanning Electron Microscope (SEM) image of universal adhesive penetration after removal of smear layer on dentin with 1000x magnification. Red arrows indicate resin tag penetration in (A) 2.5% Tamarindus indica, (B) 5% Tamarindus indica, (C) 10% Tamarindus indica, and (D) 17% EDTA groups.
| Group | Mean (SD) | p-value |
|---|---|---|
| 2.5% Tamarindus indica | 13.21 (1.5) | 0.00* |
| 5% Tamarindus indica | 13.32 (1.89) | - |
| 10% Tamarindus indica | 17 (0.99) | - |
| 17% EDTA | 12.83 (1.83) | - |
Abbreviation: SD: standard deviation.
The Bonferroni post hoc test showed that there were no significant differences in the penetration of the universal adhesive system after removal of the smear layer between 2.5% Tamarindus indica and 5% Tamarindus indica groups (p=1.00), between 2.5% Tamarindus indica and 17% EDTA groups (p=1.00), and also between 5% Tamarindus indica and 17% EDTA groups (p=1.00). Significant differences in the penetration of the universal adhesive system after removal of the smear layer were found between the 2.5% Tamarindus indica and 10% Tamarindus indica groups (p=0.00), between 5% Tamarindus indica and 10% Tamarindus indica groups (p=0.00), and also between 10% Tamarindus indica and 17% EDTA groups (p=0.00) (Table 2).
Energy Dispersive X-ray Spectrometry (EDX) analysis was used to confirm the mineral content of the specimen. EDX analysis was carried out at several points at the resin-dentin interface obtained from SEM images, and the results can be seen in Fig. (3). Calcium and phosphorus, which are the main contents of dentin, can be detected by EDX. Silica and barium were also found in the specimens tested, which confirm the filler contents of the Optibond Extra Universal adhesive system that penetrated into the dentin tubules.
Data on calcium content at the resin-dentin interface obtained from EDX analysis were then analyzed using SPSS 26.0 software. Normality and one-way ANOVA tests were then carried out. Table 3 shows that the 2.5% Tamarindus indica group had the most calcium content, 66.26 (SD 6.6) %, while the 10% Tamarindus indica group had the least calcium ion content, 44.14 (SD 11.36) %. One-way ANOVA test showed p=0.06 (p<0.05), indicating that there was no statistically significant difference in calcium ion levels in the four groups tested.
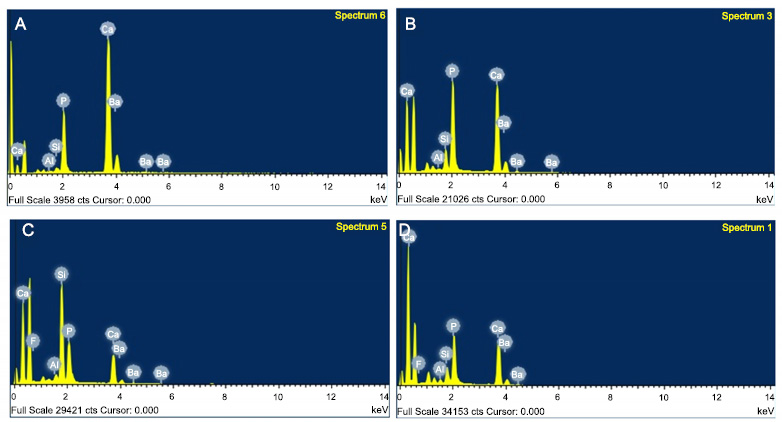
Scanning Electron Microscope (SEM) image of universal adhesive penetration after removal of smear layer on dentin with 1000x magnification. Red arrows indicate resin tag penetration in (A) 2.5% Tamarindus indica, (B) 5% Tamarindus indica, (C) 10% Tamarindus indica, and (D) 17% EDTA groups.
| - | 2.5% Tamarindus indica | 5% Tamarindus indica | 10% Tamarindus indica | 17% EDTA |
|---|---|---|---|---|
| 2.5% Tamarindus indica | - | 1.00 | 0.00* | 1.00 |
| 5% Tamarindus indica | - | - | 0.00* | 1.00 |
| 10% Tamarindus indica | - | - | - | 0.00* |
| Group |
Ca Content (%) Mean (SD) |
p-value |
|---|---|---|
| 2.5% Tamarindus indica | 66.26 (6.6) | 0.06 |
| 5% Tamarindus indica | 64.83 (16.28) | - |
| 10% Tamarindus indica | 44.14 (11.36) | - |
| 17% EDTA | 53.31 (21.76) | - |
4. DISCUSSION
The smear layer on the dentin surface causes defects in bonding, which can reduce the resistance and stability of the hybrid layer. Therefore, a self-etch adhesive system requires a mild conditioning agent as a pretreatment, which can remove the smear layer but does not damage the organic part of the dentin [16]. In this study, EDTA solution was used as a synthetic smear layer removal agent and was applied to the dentin for 60 seconds. The application of EDTA for 60 seconds can remove the smear layer, demineralize the dentin lightly, and expose collagen fibers, causing infiltration of the self-etch adhesive material into the dentin, resulting in high bond strength and long-lasting bonding [11, 17].
The natural smear layer removal agent used in this study was Tamarindus indica (tamarind). Tamarindus indica fruit extract was made into a solution with concentrations of 2.5%, 5%, and 10%. The 5% Tamarindus indica solution is acidic (pH=2), therefore it will react with hydroxyapatite and cause the dentin structure to demineralize. This mineral dissolving property allows Tamarindus indica to remove the smear layer [13].
In this study, 10% Tamarindus indica had the longest universal adhesive penetration compared to other groups (Table 1). This indicates that 10% Tamarindus indica has the lowest pH, resulting in the greatest smear layer removal, which can be seen from the longest penetration of resin tags into the dentin. A significant difference can be found in the universal adhesive penetration value between the 10% Tamarindus indica group compared to other groups. This is in line with the research by Wulandari (2012), which compared the decalcification of root canal dentin with 2.5% and 5% Tamarindus indica extracts and stated that the more acidic the material is, the more hydrogen ions that bind calcium ions, resulting in more calcium ions that are dissolved in the dentin [15]. Removal of the smear layer with the application of 2.5%, 5%, and 10% Tamarindus indica in this study was carried out for 60 seconds. There is no study examining smear layer removal with Tamarindus indica on cavities or coronal dentin; however, in the research conducted by Kumar et al. (2018), who compared smear layer removal using 5% Tamarindus indica extract with 17% EDTA on root canal dentin, stated that the application of 5% Tamarindus indica solution for 60 seconds was effective in removing the smear layer on root canal dentin [18].
Self-etch adhesive systems were developed and widely used today to overcome the postoperative sensitivity that is often encountered with the use of etch-and-rinse adhesive systems. In the etch-and-rinse system, the use of phosphoric acid before the application of the adhesive material can remove the entire smear layer and open the dentin tubules [19]. Self-etch or universal adhesive systems have been proven to be able to achieve the best dentin bond strength results without the presence of phosphoric acid etching. When the adhesive is applied directly to unetched dentin, the dentin partially demineralizes [20, 21]. Large amounts of hydroxyapatite crystals remain around the collagen fibrils. Micromechanical interactions occur due to in situ polymerization of monomers that infiltrate the collagen network. Some universal adhesive systems have functional monomers in their composition [16, 22, 23]. These monomers interact chemically with calcium in the hydroxyapatite residue via ionic bonds, thereby creating a stable insoluble nano-coating. This increases the mechanical strength and prevents degradation of the bonding interface over time [22, 24].
In the research conducted by Susin et al. (2008), specimens that were etched with phosphoric acid showed a greater depth of demineralization than those conditioned with a self-etch adhesive system and a surface free from smear layers and smear plugs, with 100% of the dentin tubules exposed [20]. The resin tags on a self-etch adhesive system were noticeably shorter than those on an etch-and-rinse [21]. Despite forming a thinner hybrid layer, the self-etch adhesive systems can provide bond strength to dentin that is comparable to or even better than the etch-and-rinse systems. A frequently encountered problem of etch-and-rinse adhesive systems is nano leakage from unprotected collagen fibrils in the hybrid layer. Phosphoric acid produces deep demineralization. This causes the adhesive monomer to not be able to infiltrate all exposed collagen fibrils [11].
The OptiBond Extra Universal adhesive system used in this research contains the functional monomer Glycerol Phosphate Dimethacrylate (GPDM), which is a short molecule with two hydrophobic methacrylate groups and one hydrophilic phosphate group, but a long carbon spacer group does not separate these functional groups as in Methacryloyloxydecyl Phosphate (MDP) [25]. Glycerol Phosphate Dimethacrylate (GPDM) was one of the first chemical compounds proposed to improve bonding with dentin [8], [26]. The shorter spacer groups and higher hydrophilicity of GPDM induce better dentin wettability compared to MDP. Adhesive materials that contain GPDM have two polymerizable groups, so they tend to react more strongly with other monomers in adhesives and restoration materials when compared to other functional monomers that have only one polymerizable group [26], [27]. A higher degree of polymerization is associated with increased quality of the polymer bond and better mechanical properties of resin-based materials [27]. GPDM functional monomers have one phosphate group [8]. This phosphate group can bond with demineralized dentin. Phosphate is an unstable ion. Hence, demineralization causes the phosphate to be lost from dentin. Therefore, the bond that occurs between GPDM monomers that contain phosphate groups and calcium in dentin can increase the adaptation of this universal adhesive material to the dentin surface [28].
In this study, specimens were tested using SEM (Scanning Electron Microscope). Scanning Electron Microscope is an effective test tool for evaluating the adhesive material-dentin interface. The image of the adhesive material-dentin interface (Fig. 2) shows the presence of a thin but clearly visible hybrid layer, with the presence of resin tags. The role of resin tags in the bonding mechanism of self-etching adhesive materials is still debated. A study by Lohbauer et al stated that there was no effect of resin tags on the bond strength of self-etch adhesive materials because micro-tensile bond strength could decrease with or without the presence of resin tags after thermocycling [29]. Resin tags produced from etch-and-rinse adhesive systems showed high penetration depth. Long resin tags provide good mechanical retention of resin to dentin thus it can increase the immediate bond strength [30].
In this study, SEM images showed that a thin layer of adhesive material was found in the 2.5% and 5% Tamarindus indica groups (Fig. 2a and b). A thicker adhesive layer can be observed in 10% Tamarindus indica and 17% EDTA groups (Fig. 2c and d). The thickness of the adhesive layer indicates the material's ability to clean the smear layer. A thicker adhesive layer indicates better smear layer removal. However, the research conducted by Kharouf (2021), which compared the thickness of several universal adhesive materials on immediate and long-term bond strength, showed that the thickness of the adhesive layer did not influence the shear bond strength. The bond strength of the resin-dentin interface does not depend on the thickness of the hybrid layer and/or the adhesive system. A thick adhesive layer does not provide an increase in bond strength, while the quality of the hybrid layer is an important factor that can influence the bond strength of dentin [31].
Scanning Electron Microscope (SEM) can be combined with X-ray microanalysis called Energy Dispersive X-ray Spectrometry (EDX). This EDX test is used to improve the topographic analysis of specimens with additional information on their elemental composition [32]. In this study, EDX was taken from SEM images at the adhesive-dentin interface to confirm the penetration of universal adhesive system into dentin tubules (Fig. 3). Measurement of calcium ion content in this study (Table 3) shows that there is no statistically significant difference in calcium ion levels between the four groups tested. This shows that Tamarindus indica and EDTA can remove the smear layer on dentin, but will not lose its inorganic substrate, calcium. The remaining calcium mineral content around the collagen fibrils is needed to form chemical bonds with the phosphate or carboxylic groups in the functional monomers found in self-etch adhesive systems. This chemical bond reduces hydraulic degeneration and maintains the marginal sealing of the restoration for a longer period of time [33].
CONCLUSION
The application of 10% Tamarindus indica solution was effective in the removal of the smear layer on dentin and resulted in longer penetration of resin tags compared to 2.5% Tamarindus indica, 5% Tamarindus indica, and 17% EDTA.
AUTHORS’ CONTRIBUTION
It is hereby acknowledged that all authors have accepted responsibility for the manuscript's content and consented to its submission. They have meticulously reviewed all results and unanimously approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| CEJ | = Cemento-enamel Junction |
| EDTA | = Ethylenediamine Tetra Acetic Acid |
| EDX | = Energy Dispersive X-ray Spectrometry |
| GPDM | = Glycerol Phosphate Dimethacrylate |
| MDP | = Methacryloyloxydecyl Phosphate |
| PBS | = Phosphate Buffered Saline |
| SE | = Self-etch |
| SEM | = Scanning Electron Microscope |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study has obtained ethical approval from the Dental Research Committee, Faculty of Dentistry, Universitas Indonesia (ethical clearance number: 11/Ethical Approval/FKGUI/III/2023).
HUMAN AND ANIMAL RIGHTS
All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committees and with the 1975 declaration of Helsinki, as revised in 2013.
AVAILABILITY OF DATA AND MATERIALS
The authors confirm that the data supporting the findings of this study are available within the article.


