All published articles of this journal are available on ScienceDirect.
The Antifungal Effects of Tea Tree Oil as a Potential Therapeutic Candidate for Candida-related Infections in Albino Rats
Abstract
Background
Fungal infection is an irritating problem because of the limited number of antifungal drugs and their adverse reactions. Moreover, in the past few years, the resistance of Candida to the existing antifungals has been observed.
Methods
The study sample consisted of 60 male albino rats. Group I received a physiological solution. Group II was subjected to systemic antibiotic treatment 1 week before the application of Candida infused on the tongue dorsum. Group III was subjected to systemic antibiotics, as was group II, for 3 consecutive days, and tea tree oil was applied throughout the experimental period. Ten rats from each group were sacrificed at five and seven weeks and tongue specimens were dissected and prepared for histological and transmission electron microscopic examination.
Results
Histological and TEM results in group II after five and seven weeks revealed marked degenerative changes in the dorsal surface of the rat tongue. Nonetheless, in group III, obvious regeneration of epithelial tongue tissue appeared after seven weeks of treatment with oil.
Conclusion
Tree oil showed antifungal properties against Candida infection, which was confirmed by ultrastructural examination.
1. INTRODUCTION
Microorganisms residing in the oral cavity are significant components in altering the balance between health and sickness and include several hundred to thousands of diverse species. The mouth contains bacteria, archaea, protozoa, fungi, and viruses, although each have their own unique characteristic known as a “fingerprint,” usually living in symbiotic harmony with the host [1].
Candidiasis/ moniliasis is a versatile fungal microbe which occurs as a result of the commensalism Candida species, which inhabit the epidermis, integumentary systems, and alimentary canal of 30–50% of physically fit grown up at any time during the course of life [2]. During the normal environment, Candida is not generally infective and mostly resides in the vulva and alimentary canal in humans with good physical conditions. However, candidiasis occurs when the equilibrium between the mucosa, mycelium, and host resistance has been distressed [3]. Candida is ascertained to cause exterior disease (mucous membrane and skin) and interior disease. In addition to oral and vaginal, exterior diseases appear in different sites, and they can also be seen in oropharyngeal and onychomycosis, etc., with widespread occurrence (20–25%) [4].
Given that the upper surface of the tongue is the main environment in the mouth of physically fit and immuno- deficient human beings, most academic works on investi- gational oral candidiasis in model organisms were carried out on the tongue’s upper surface [5]. The tongue of rodents is a fibrous organ covered by cornified stratified squamous epithelium, which constitutes the top portion which is known as the dorsum, and the bottom portion, called the ventral. Below the epithelium, there is connective tissue, which is heavily loaded with blood vessels and skeletal muscle. In this area, fibers are assembled into bundles in three planes. Serous and mucous salivary glands are present in the posterior region between the muscle cells [6].
Comparable to the human tongue's upper surface, the rat and mouse tongues have many protrusions called filiform/threadlike and fungiform papillae. Filiform papi- llae are categorized as uncomplicated conical growth (positioned on the anterior 2/3 of the tongue), true papillae (situated on the intermolar tubercle), and giant papillae (found between the simple conical and the true papillae). In between the group of uncomplicated conical projections, there are a small number of fungiform papillae, which are stubby and wide with a circular base that includes the savor unit in the epithelium of the top side [6].
A great number of medicinal plants have been used as antimycotic medications, including tea tree oil, water like margosa concentrate, and gum tree essential oil [7, 8]. The tea tree is generally called Melaleuca alternifolia (M. alternifolia)/melaleuca oil. It demonstrates a wide range of antibacterial actions and is efficient in anticipation of further harmful yeasts and catalase-positive and disconfirming negative bacteria [9].
Tea tree oil (TTO) is a combination of essential oils (volatile oils, ethereal oils, aetheroleum), including approximately 100 ingredients, the majority of them are monoterpenes, sesquiterpenes, with their related alcohols [9]. TTO has been verified to have a number of curing effects while acting as an inflammation reducer [10], and there is ongoing research on its attainable anti-tumor properties [11]. Nevertheless, it is distinguished for its biocidal effects that are antagonistic to an extended range of pathogens, for instance, Staphylococcus aureus (including methicillin-resistant Staphylococcus aureus), [12], a variety of oral bacteria [13], and specific types of viruses, i.e., herpes simplex and influenza viruses [14]. Moreover, TTO has powerful action as opposed to numerous mycelium [15], and there isand there is confirmation of its benefit in managing fluconazole refractory oral candidiasis in HIV-positive patients has been confirmed [16]. Such properties increase the chance of utilizing TTO for avoiding and treating oral candidal infections [17].
Hence, this study aimed to conduct an ultrastructural evaluation of TTO as an antifungal agent for the treatment of candidiasis induced in the tongues of albino rats.
2. MATERIALS AND METHODS
All investigational steps were approved by the Institutional Animal Care at the Faculty of Dentistry, Mansoura University, Egypt (approval No. A 11070837). This research was conducted in compliance with the ARRIVE (Animal Research: Reporting of in Vivo Experiments) guidelines and regulations (https://arrive guidelines.org).
Sixty mature male rats weighing 120-150gm were utilized in this research, purchased from the authorized bio base of the faculty of Medicine, Mansoura University. The rats were confined in specially designed wire mesh bottom cages, 5 rats per cage, to maintain them under the most appropriate conditions of good aeration and the rats were supplied with a diet composed of rough corn, barley, and powdered milk in addition to fresh vegetables, and they drank tap water throughout the whole seven weeks of the experimental period. The rats were maintained in an animal health care facility under the supervision of the local ethical committee in a laboratory animal colony.
A priori analysis was conducted, which is a sample size calculation performed before conducting the study and before the design and planning stage of the study; thus, it is used to calculate the sample size N, which is necessary to determine the effect size, the desired α level, and power level (1-β).
2.1. Inclusion Criteria
The selection of the animal models for research was based on the following considerations: 1) appropriateness as an analog, 2) transferability of information, 3) genetic uniformity of organisms, where applicable, 4) background knowledge of biological properties, and 5) cost and availability.
The rats were divided into three equal groups (n = 20):
2.1.1. Group I
The rats were served as controls, given isotonic saline in the matching cubic measure and repetitiveness as groups II and III.
2.1.2. Group II
The rats were given systemic antibiotic treatment 1 week before the application of C. albicans infused on the tongue dorsum [18].
2.1.3. Group III
In this group, rats were given systemic antibiotic treatment 1 week before the application of C. albicans infused on the tongue dorsum and then given tea tree oil 1% Tween 80, applied to the tongue dorsal surface with feeding needles 4 days after infection by C. albicans and continued throughout the experimental period [19].
The rats of groups II and III were given 14 mg/kg body weight of augmentin (amoxicillin and clavulanic acid) and Amoxyclav, Manufacturer. Glaxo SmithKline Pharma- ceuticals, in their drinking water twice a day once a week before oral inoculation with C. albicans, and they continued to receive antibiotic treatment over the experimental period [18].
A suspension of C. albicans, purchased from the Centraal Bureau voor Schimmel cultures ([CBS], Utrecht, the Netherlands) [20], was prepared to get the eventual concentration of 1-5 × 103 cells/mL, according to (NCCLS, 2002) [21]. The rats were put under sedation by injection within the muscle in each foot with 50 ml of 0.2% chlorpromazine chloride (Wako Pure Chemical Industries, Ltd., Osaka, Japan) [22]. C. albicans suspension (0.2 ml) was poured into the rats' mouth with the aid of a 1 ml syringe and a 30×8 mm blunt needle. Additionally, the material was straightened out on the tongue dorsum with a sponge formerly absorbed suspension. All rats were not given water for at minimum of 1 h after infusion with Candida. This series of steps was repeated for 3 successive days [23].
Furthermore, to evaluate the establishment of C. albicans in the rats' mouth, CFU (colony-forming unit) sum-up is the most advantageous method. Specimens from the surface of the tongue were collected 6 days after inoculation, using a swab for rear CFU counting onto Sabouraud dextrose agar (SDA) with antibiotics. This method can estimate if the rats were colonized by C. albicans and the antimycotic effects of the tested remedial approach [24].
Tea tree oil obtained from Sigma-Aldrich (Poole, Dorset) was used in the study. The MICs (minimum inhibitory concentrations) of TTO against C. albicans were set by using the broth microdilution method (M27-P protocol) as reported by the National Committee for Clinical Laboratory Standards (NCCLS) [21]. TTO was suspended in 1% Tween 80 and applied to a cotton swab 4 days after C. albicans inoculation and was continued through- out the experimental period. This oral application scheme was proposed by former therapeutic studies of farnesol, a quorum-sensing molecule [25].
Ten rats of each group were euthanized by cervical dislocation after five and seven weeks from the start of the experiment. The tongue specimens were dissected from each rat. Each specimen was divided into right and left halves. The Specimens of the right side were prepared for trans- mission electron microscope (Thermo Fisher Scientific Inc. Hitachi High-Tech America, Inc. USA), using autoscript 4 software [26] to examine all epithelial layers (keratin, granular, spinous, and basal cell layer). In contrast, those of the left side were prepared for histological staining.
2.2. Exclusion Criteria
The animals were excluded if complications were anticipated during tongue dissection or there was a failure to meet quality control standards, such as unacceptable levels of contaminants or poor histological quality
2.2.1. Staining
Hematoxylin and Eosin staining is a routine histological staining procedure. For microscopic analysis of the specimens, the tongues were fixed in 10% formalin for 24 h. After embedding in paraffin, 5 μm-thick tissue slices were cut and stained with hematoxylin-eosin (H&E). The presence of candidiasis was analyzed using optical microscopy (Olympus, CX41, Toquio, Japan) at X100 magnification with ZEN End-to-end microscopy software [27].
These histological sections were investigated under an optical light microscope.
The summary of the followed protocol in the study is as follows [18, 19]:
2.2.1.1. 1st Week
Augmentin treatment, was continued until the end of the experiment (7th week) [18].
2.2.1.3. 3rd Week
Starting of TTO treatment and continuation till the end of the experiment (7th week) [19]
3. RESULTS
Intra orally, small white blotches of “thrush” were observed on the keratinized tongue mucosa and occasionally on the buccal mucosa.
3.1. Light Microscopic Results
3.1.1. Hematoxylin and Eosin Stain
The histological examination revealed the following:
3.1.1.1. Control Group (Group I)
The control section of the tongue showed normal thread-like keratinized filiform papillae with the normal arrangement of the four epithelial layers. Each layer could be distinguished from the others. The connective tissue showed a normal arrangement of fibroblasts and collagen, as shown in Fig. (1A).
3.1.1.2. Group II (systemic Antibiotic Treatment and Oral Infusion of C. Albicans on the Tongue Dorsum)
After five weeks, great atrophy of lingual papillae was observed with massive infiltration of entangled accumulation of candidal leaves infiltrating the superficial layers of the atrophied lingual epithelium, forming extensive intraepithelial microabscesses with hyper- keratosis. The submucosa with its connective tissue papilla showed individual accumulation of inflammatory cells with distended blood vessels, as shown in Fig. (1B, C).
After seven weeks, complete loss of filiform papilla with partial disappearance of candidal yeasts was observed. The lingual epithelium showed extensive epithelial hyperplasia with obvious enlarged rete pegs, keratin plugging, partial absence of granular cell layer, acanthosis of the prickle cell layer, and disordered, packed basal cell layer, as shown in Fig. (1D).
3.1.1.3. Group III (Systemic Antibiotic Treatment and Oral Infusion of C. Albicans on the Tongue Dorsum, with later Introduction of TTO):
After five weeks, noticeable lingual papillary atrophy was observed with massive infiltration of entangled accumulation of Candida leaves in the superficial layers of the atrophied lingual epithelium, forming extensive intraepithelial micro abscesses accompanied with hyperkeratosis, as shown in Fig. (1C).
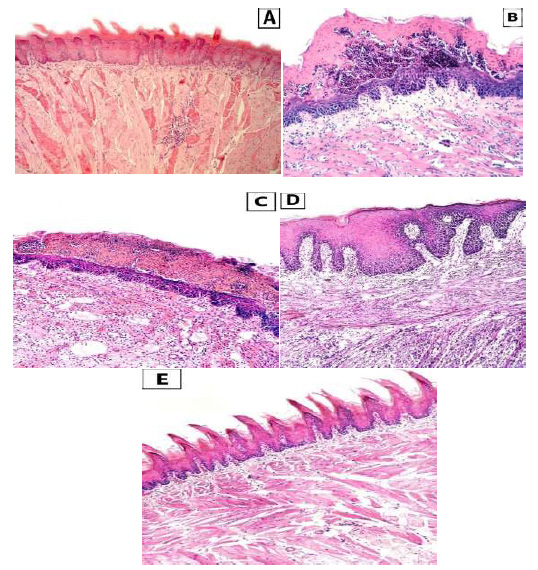
Showing Light microscope photomicrograph of the three groups (A): showed the lingual mucosa of group I with normal stratified squamous epithelium. (B) and (C) showed Group II & III respectively after 5 weeks showing massive candidal invasion with great lingual papillary atrophy. The lamina propria and the muscular layer are markedly accompanied with inflammatory cell infiltrate. (D) and (E) Group II& III respectively after 7 weeks, group II showing partial disappearance of candidal invasion and extensive epithelial hyperplasia with loss of filiform papillae and obvious elongated, enlarged rete pegs. The lamina propria and the muscular layer are markedly accompanied with inflammatory cell infiltrate, while in group III showing complete disappearance of candidal invasion and normally appeared thread like keratinized filiform papillae with equal thickness of the lingual epithelium and no elongation in the rete pegs. The lamina proropria and the muscular layer appear with normal architecture (H&E stain x 100).
After seven weeks,normal thread-like keratinized filiform papilla was observed, and candidal yeasts completely disappeared. The lingual epithelium showed a keratin layer with normal thickness of all layers. The submucosa, with its connective tissue papilla, presented a regular arrangement of fibroblasts and their collagen fibers with no inflammatory cells, as shown in Fig. (1E).
3.2. Electron Microscopic Results
3.2.1. Control Group (Group I)
After five weeks, different layers of the lingual epithelium showed a normal intact keratin layer, as shown in Fig. (2A-C). Normal appearance of granular cells was observed with euchromatic nuclei and evident nucleoli, numerous tonofilaments in the cytoplasm, and intact desmosomal junctions, as shown in Fig. (2D). The normal appearance of spinous cells was observed with euchromatic nuclei and intact desmosomal junctions, as shown in Fig. (2G).
Normal appearance of the basal cell layer was also observed with clear evident nuclei with normal chromatin distribution and intact basal lamina, as shown in Fig. (2J).
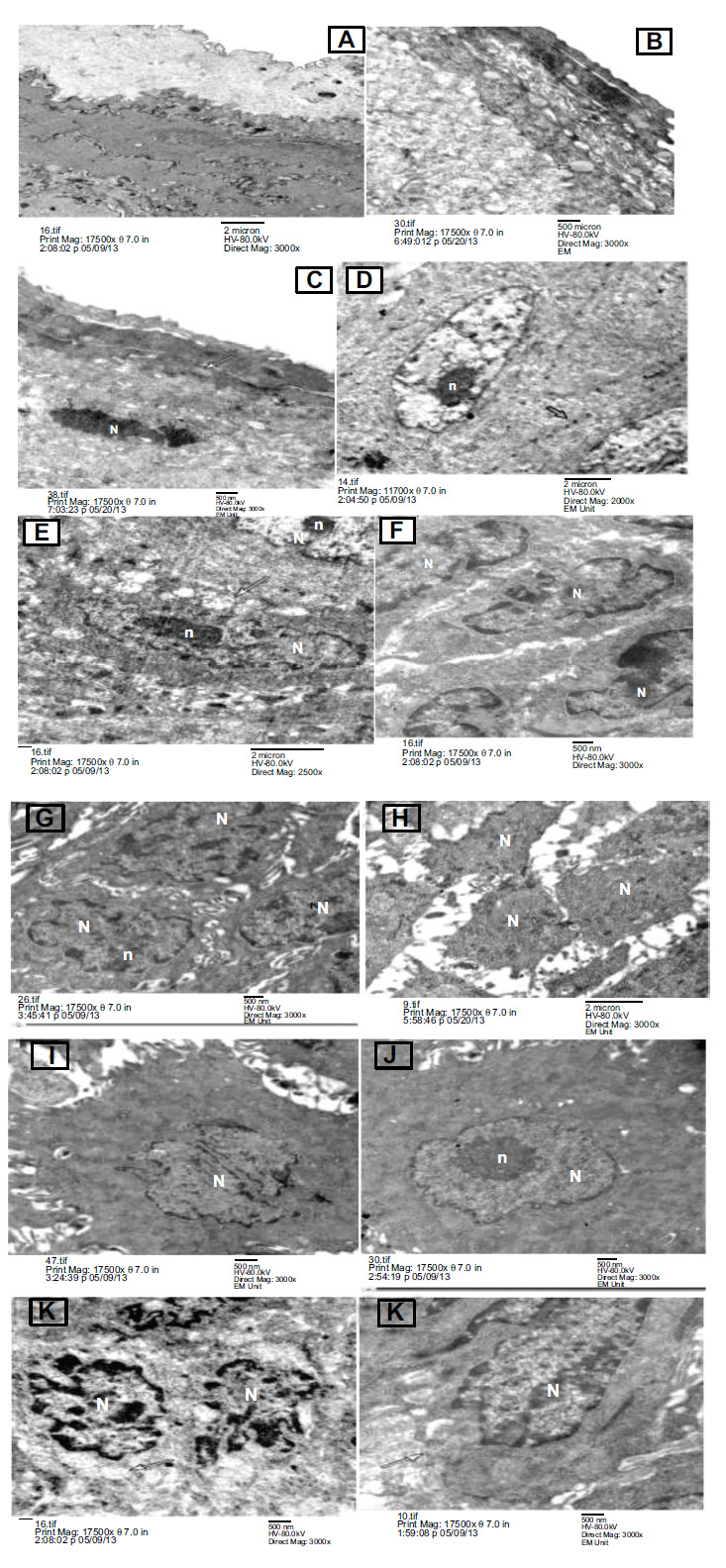
Showing Transmission electron micrograph of the three group after 5 weeks (A) group I control group showing normal intact keratin layer. (B) and (C) showed Group II & III respectively at 5 weeks, showing Candida albicans yeasts appear invading the keratin layer (spherical blastospores) (arrow). (D) control group showing, normal looking granular cells with euchromatic nuclei (N) and evident nucleoli (n),numerous tonofilaments in the cytoplasm and intact appearing desmosomal junctions (arrow), (E) and (F) Group II& III respectively after 5 weeks, group II showing granular cell appear with swollen mitochondria (arrow) and destructed desmosomal junctions, while in group III showing granular cells appear with widened intercellular spaces and destructed desmosomal junctions, (G) control group showing, normal looking spinous cells with euchromatic nuclei (N) and evident nucleoli (n) and intact desmosomal junctions, (H) and (I) group II& III respectively showing spinous cells appear with abnormal nuclei (N), severely widened intercellular spaces and destructed desmosomal junctions, (J) group I control group showing, normal looking basal cells. The nuclei (N) are clear evident with nucleoli (n) and normal chromatin distribution (K) group II showing basal cells appear with abnormal heterochromatic nuclei (N) and swollen mitochondria (arrow), (L) group III showing basal cell appears with destructed desmosomal junctions and irregular basal lamina (arrow). (EM x17500).
After seven weeks, different layers of the lingual epithelium showed normal intact keratin layers, as shown in Fig. (3A-L). Normal appearance of granular cells was observed with euchromatic nuclei, evident nucleoli, and intact desmosomal junctions, as shown in Fig. (3D). The normal appearance of spinous cells was observed with euchromatic nuclei and intact desmosomal junctions, as shown in Fig. (3G). The normal appearance of the basal cell layer was also observed with clear evident nuclei with normal chromatin distribution, as shown in Fig. (3J).
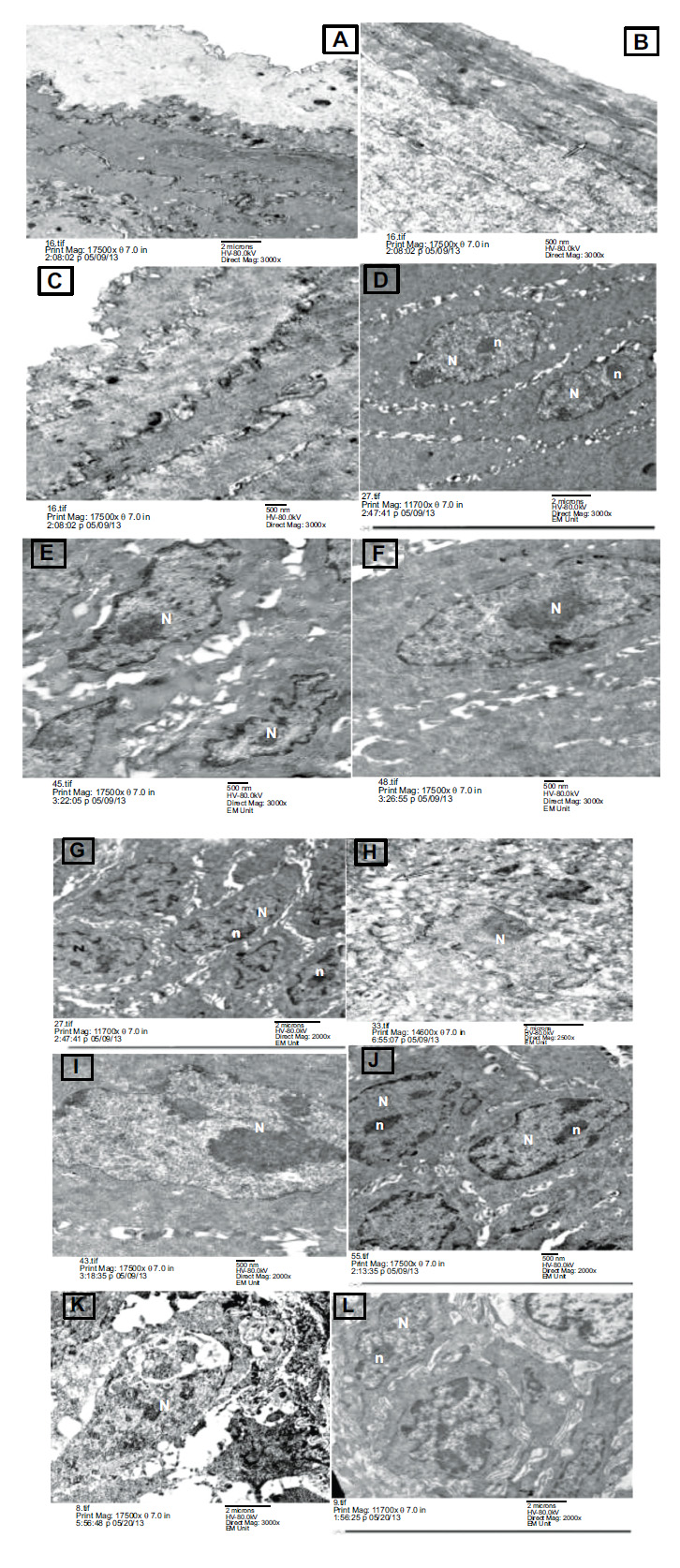
Transmission electron micrograph of the three group after 7 weeks (A) group I control group showing normal intact keratin layer. (B) and (C) showed Group II & III respectively after 7 weeks, group II still showing Candida albican yeasts appear invading the keratin layer (spherical blastospores) (arrow) while in group III showing Healthy looking intact keratin layer appears with complete disappearance of candida yeasts. (D) control group showing, normal looking granular cells with euchromatic nuclei (N) and evident nucleoli (n), numerous tonofilaments in the cytoplasm and intact appearing intercellular junctions, (E) and (F) Group II& III respectively after 7 weeks, group II showing granular cells appear with widened intercellular spaces and destructed desmosomal junctions, while in group III showing granular cell appears with euchromatic nucleus (N) and intact desmosomal junctions, (G) control group showing, normal looking spinous cells with euchromatic nuclei (N) and evident nucleoli (n) and intact intercellular junctions , (H) and (I) group II& III respectively, group II showing spinous cells appear with abnormal nuclei (N), severely widened intercellular spaces and destructed desmosomal junctions, While in group III showing spinous cells appear with euchromatic nucleus (N) and intact desmosomal junctions (J) group I control group showing, normal looking basal cells. The nuclei (N) are clearly evident with nucleoli (n) and normal chromatin distribution, (K) group II showing basal cells with abnormal nucleus (N), severely widened intercellular spaces and destructed desmosomal junctions), while in (L) group III showing basal cells appear with euchromatic nuclei (N) and intact desmosomal junctions. (EM x17500).
3.2.1.1. Group II (Systemic Antibiotic Treatment and Oral Infusion of C. Albicans on the Tongue Dorsum)
After five weeks, Candida albicans’ yeasts appeared to invade the keratin layer (spherical blastospores) with separation of the superficial layer, as shown in Fig. (2B).
Granular cells emerged with severely widened intercellular spaces and destructed desmosomal junctions, as shown in Fig. (2E). Spinous cells were seen with abnormal nuclei, severely widened intercellular spaces, and destructed desmosomal junctions, as shown in Fig. (2H).
Basal cells appeared with abnormal nuclei, severely widened intercellular spaces, and destructed desmosomal junctions, as shown in Fig. (2K).
After seven weeks, Candida albicans yeasts appeared to invade the keratin layer (spherical blastospores), as shown in Fig. (3B). Granular cells emerged with widened intercellular spaces and destructed desmosomal junctions, as shown in Fig. (3E).
Spinous cells were seen with swollen mitochondria and destructed desmosomal junctions, as shown in Fig. (3H).
Basal cells appeared with pyknotic nuclei and destructed desmosomal junctions, as shown in Fig. (3K).
3.3. Group III (Systemic Antibiotic Treatment and Oral Infusion of C. Albicans on the Tongue Dorsum, with later Introduction of TTO )
3.3.1. After Five Weeks
Candida albican yeasts appeared to invade the keratin layer (spherical blastospores), as shown in Fig. (2C). Granular cells emerged with broadened intercellular spaces and destructed desmosomal junctions, as shown in Fig. (2F).
Spinous cells emerged with broadened intercellular spaces and destructed desmosomal junctions, as shown in Fig. (2I).
Basal cells appeared with destructed desmosomal junctions and irregular basal lamina, as shown in Fig. (2L).
3.3.2. After Seven Weeks
A healthy-looking intact keratin layer appeared with the complete disappearance of candidal yeasts, as shown in Fig. (3C).
Granular cells appeared with a euchromatic nucleus and intact desmosomal junctions, as shown in Fig. (3F).
Spinous cells appeared with a euchromatic nucleus and intact desmosomal junctions, as shown in Fig. (3I).
Basal cells appeared with euchromatic nuclei and intact desmosomal junctions, as shown in Fig. (3L).
4. DISCUSSION
Mycosis infections are increasing as a result of their dissemination within society, their long life, the use of antirejection drugs, diabetes mellitus, obesity, excessive sweating, and Subungual hematoma [28]. Further, there are many drawbacks during the intervention to treat these infections intraorally, such as a rise in the counteraction to the antifungal drugs as a result of its utilization above the normal level, pharmacodynamical interactions with other therapeutics, and finally, its unfavorable side effects like the possibility of liver disease. As a consequence, the usage of antifungal drugs has become restricted [29]. In addition, oral antimycotic remedial treatment can influence the drug metabolism of formerly advised medi- caments and may alternate their outcomes, decrease their competence, or finally elevate their toxicity [30]. As a consequence of these issues, a more relevant policy was grown as an alternative therapy to ordinary treatment. Surrogate remedies are currently under consideration, incorporating the utilization of essential oils (EO) as an attainable antifungal drug [29].
The immune response of the rats was reduced by giving them with 14 mg/kg body weight of augmentin (Amoxicillin and Clavulanic acid) in their drinking water twice a day one week before oral inoculation with C. albicans. This process was continued over the experimental period to eliminate bacteria in the oral cavity and contribute to the growth of C. albicans leading to the failure of the immune system to obliterate the adherence of candida, which produces oral candidiasis. Candidiasis activates the distinction of T helper (Th) cells to Th17 to manufacture Interleukin 17 (IL- 17), which induces induces the gathering and antimycotic effect of neutrophils [31].
The use of a calmative means for the rats during C. albicans inoculation was essential because the infusion of Candida intraorally without any anesthetic drug diminished the expansion of Candida and, later on, the occurrence of candidiasis. Based on the findings of Takakura et al. [22], the severity of the infection corresponds to the length of the narcotized period. The calmness lessens the extension of candida to the esophagus and stomach and advocates a better establishment and tissue infiltration by candida.
In the histologic results, it was observed that candidiasis appeared on the dorsum of the rats’ tongue in group II (after 5 and 7 weeks) and in group III (after 5 weeks) as extensive epithelial hyperplasia with loss of filiform papillae, and the lamina propria, and the muscular layer were markedly invaded by inflammatory cell infiltrate, and this was in accordance with the findings of Junqueira JC et al., 2009, who observed the occurrence of an increased number of epithelial cells relative to the basement membrane, disordered basal cell layer, exocytosis, intercellular edema, increased cellular division in stratum germi- nativum, the disappearance of filiform papillae, hyper parakeratosis, and the invasion of inflammatory cells in the connective tissue was also reported [32].
Moreover, Costa et al., 2013, reported the same findings when they investigated oral candidiasis in a mouse and rat model where an increased number of epithelial cells relative to the basement membrane, disordered basal cell layer, exocytosis, intercellular edema, degeneration of filiform papillae and hyper- parakeratosis was also reported [33].
Several studies have shown the antimycotic efficacy of different trading EO in vitro [34] by measuring (MIC), i.e., the lowest concentration of an antimicrobial that will inhibit the visible growth of a microorganism after overnight incubation, and (MFC), i.e., the lowest concentration of monoterpenes resulting in the death of 99.9% of the inoculum [29].
In a research, M. alternifolia was suspended in 1% Tween 80 to be straightened out on the superior surface of the rats’ tongues in group III. Tween 80 (polyethylene sorbitol ester), a nonionic surface-active agent and an emulgent, was regularly utilized as a conveyor for M. alternifolia [35]. Many researches have stated that Tween 80 has a lower lethal effect on human and animal cells. Moreover, its implementation is assent for injection either in the skin or in the veins of humankind [36].
In the current investigation, M. alternifolia was used 4 days after Candida infusion. Therefore, candidiasis was highly confirmed, resulting in the impediment of M. alternifolia action. Additionally, candidiasis was examined on the dorsum tongue 2 weeks after the introduction of M. alternifolia, and this duration may not have been sufficient for the advancement of the lesions and tissue repair to be observed [19]. The findings were in the form of invasion of Candida albican yeasts to the keratin layer (spherical blastospores), and granular and spinous cells appeared with widened intercellular spaces and destructed desmosomal junctions and basal cells appeared with destructed desmosomal junctions and irregular basal lamina; these observations in agreement with Juliana et al., 2005 who used scanning electron microscope to explore the effect of experimental candidiasis in ovariectomized rats. They verified the atrophy of filiform papillae and the increase in interpapillary surface [27].
After 4 weeks of treatment with TTO, tissue improvements were more obvious when seen by transmission electron microscope in all layers of epithelium, and a healthy intact keratin layer appeared with complete disappearance of candidal yeasts; granular, spinous, and basal cells appeared with euchromatic nucleus and intact desmosomal junctions, indicating the tissue enhancement produced by application of TTO. Some investigators proved the antifungal effect of TTO, such as Wen-Ru et al., 2016, who studied the kinetics and techniques of the fungi static and bacteriostatic action of tea tree oil by using transmission electron microscopy and asserted that TTO pierced the cell wall and plasma membrane of bacterial and fungal strains which were tested (including candida albicans), meaning that TTO may produce its antimicrobial effects by disrupting the cell membrane, leading to loss of the cytoplasm and cell organ damage, which ultimately leads to necrosis [37].
The TTO component, terpinen-4-ol, is the main effective ingredient and accounts for its antimicrobial potency as it can adhere to the fat-loving components of microorganisms, such as the plasma membrane, resulting in increased permeability and loss of the essential electrolytes needed for cell survival. In accordance with. Carson et al. [9], using Melaleuca alternifolia oil as a treatment to generates changes in mitochondrial membranes for 95% of Candida albicans, Candida glabrata, and Saccharomyces cerevisiae by glucose-induced acidi- fication. The acidification occurs to push out the plasma membrane proton ATPase, which is powered by ATP derived from mitochondria. The obstruction of this function indicates the deleterious effect on plasma and/or mitochondrial membranes; however, it should be considered that other recently introduced compounds have been demonstrated to have a significant influence on the oral environment. The use of postbiotics [38], lysates [39], and Paraprobiotics [40] can modify clinical parameters in periodontal patients, Therefore these products should be considered in future research in combination with Tea Tree Oil in order to evaluate their mutual effect for candida-related infection.
Using essential oils (EO), obtained from other sources, such as thyme and cinnamon, is a possible alternative therapy with empirical evidence of good results. The antifungal effects of EO may be a very promising solution to overcome the therapeutic shortcomings of antimycotic medication, which are increasing with immunosuppressive treatments and the appearance of resistant strains, among other factors. Some studies have highlighted the fungicidal action of EO against diseases caused by fungi in humans [41]. Another treatment alternative is the combination of terpinen-4-ol and nystatin, which has a potential antifungal effect on the two Candida species (Candida albicans and Candida tropicalis), showing synergistic and additive effects on planktonic and biofilm cultures [42].
CONCLUSION
In summary, our ultrastructural study of the dorsal epithelium of the rat tongue clarified that TTO has potential therapeutic and irreversible mediated actions against Candida albican strains due to its ability to invade the cell wall and cytoplasmic membrane, causing destruction to these structures, with subsequent loss of cytoplasmic material and cell death.
LIMITATIONS OF THE STUDY
The present research was conducted on an animal experimental model; although such a model provides invaluable assistance, it is not entitled to its direct impact on humans. Moreover, further studies are warranted to examine the pharmacokinetic and toxicological behaviors of TTO and the properties to devise its use as an effective treatment for Candida.
CLINICAL RELEVANCE
Melaleuca alternifolia (tea tree) was stated to have an antimicrobial effect against a broad range of gram-positive and gram-negative bacteria, yeasts, and fungi. In the present study, the antifungal actions of tea tree oil on candidiasis infection were studied by using the transmission electron microscope.
PRACTICAL IMPLICATIONS
This research investigated the antimycotic action of tea tree oil as a possible therapeutic candidate for Candida-related infections in albino rats using a transmission electron microscope.
AUTHORS’ CONTRIBUTION
Amira A.R. Moawad and Sally H. Abo Baker carried out the experimental procedures and wrote the manuscript. All authors read and approved the final manuscript.
LIST OF ABBREVIATIONS
| TEM | = Transmission electron microscope |
| TTO | = Tea tree oil |
| CFU | = Colony-forming unit |
| SDA | = Sabouraud dextrose agar |
| NCCLS | = National Committee for Clinical Laboratory Standards |
| H&E | = Hematoxylin and eosin |
| EO | = Essential oils |
| MIC | = Minimum inhibitory concentration |
| MFC | = Minimum fungicidal concentration |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All investigational steps were approved by the Institutional Animal Care at the Faculty of Dentistry, Mansoura University, Egypt (approval No. A 11070837).
HUMAN AND ANIMAL RIGHTS
This study adheres to internationally accepted standards for animal research, following the 3Rs principle. The ARRIVE guidelines were employed for reporting experiments involving live animals, promoting ethical research practices.


