All published articles of this journal are available on ScienceDirect.
Comparison of the Antibacterial Activity of Panax Ginseng and Symphytum Officinale with Metronidazole against P. gingivalis: An MIC and MBC Analysis
Abstract
Background
The RapID ANA II panel was used to evaluate bacterial responses, and the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) were established.
Aim
This study aimed to investigate the antibacterial effects of Metronidazole, Symphytum Officinale, and Panax Ginseng on P. gingivalis
Materials and Methods
P. gingivalis strains, including strain ATCC 33277 and an isolate, were selected and prepared. A variety of test compounds, including Metronidazole, Symphytum Officinale, and Panax Ginseng, were procured and manufactured. A consistent technique was used to determine the MIC and MBC. We used the RapID ANA II panel to assess bacterial responses. Additionally, a suitable software was used to conduct the statistical analysis.
Results
The findings from the MIC and MBC tests showed notable variations and a noticeable impact of the combination therapy (G+F and S+F) in comparison to the individual drugs alone. Lower MIC and MBC values were seen when Panax Ginseng and Metronidazole (G+F) and Symphytum Officinale and Metronidazole (S+F) were combined, demonstrating a synergistic effect (p < 0.01). Positive results were found for ρ-Nitrophenyl-β, D-disaccharide (BLTS), σ-Nitrophenyl-β, D-galactoside (ONPG), ρ-Nitrophenyl-n-acetyl-β, D glucosaminide (NAG), ρ-Nitrophenylphosphate (PO4), Phenylalanine-β-naphthylamide (PAL), Pyrrolidonyl-β-naphthylamide (PYR), and Tryptophane (IND) in the RapID ANA II panel, whereas negative results were obtained for Urea (URE), ρ-Nitrophenyl-α, L-arabinoside (aARA), ρ-Nitrophenyl-α, D-glucoside (aGLU), ρ-Nitrophenyl-β, D-glucoside (BGLU), ρ-Nitrophenyl-α, D-galactoside (aGAL), ρ-Nitrophenyl-α, L-fucoside (aFUC), Proline-β-naphthylamide (PRO), and Serine-β-naphthylamide (SER).
Conclusion
Our study shows that combination therapies using Panax Ginseng and Symphytum Officnale with Metronidazole have increased antibacterial efficacy against P. gingivalis. These results point to the possibility of these herbal remedies complementing conventional medicine. In this study, the RapID ANA II panel helped identify and characterise P. gingivalis by offering useful insights into bacterial reactions. Nonetheless, it is necessary to conduct more studies to examine the therapeutic uses of these alternative P. gingivalis infection treatments.
1. INTRODUCTION
Periodontitis is a chronic, inflammatory condition that affects the tissues around the teeth and usually develops while the body fights an infection [1]. The severity of the illness increases as the inflammation worsens [2]. Certain factors may affect this inflammation, speeding up the degeneration of the tissue [3, 4]. A biofilm is made up of several bacterial cells clumped together on an extracellular mucosa, which is made possible by the bacteria's production of a matrix of lipids, proteins, and polysaccharides.
When bacteria are exposed to severe environmental factors, such as excessive food deficiency or abundance, high osmotic pressure, low pH, oxidative stress, antibiotics, and antimicrobial agents, they frequently form biofilms [5]. The mainstay of treatment for bacterial infections is antibiotics [6], but since biofilms protect bacterial cells, medicines are less effective, leading to persistent infections [7]. It is difficult to control bacterial infections using conventional antibiotics because bacteria within a biofilm can become approximately 1000 times more resistant to them [8]. Therefore, it is imperative to discover methods to prevent biofilm formation in order to control these severe illnesses.
Quorum sensing (QS), a technique used by bacteria to create biofilms, entails bacterial communication utilising tiny, diffuse chemical signalling molecules known as autoinducers (Ais). These signalling chemicals build up in the environment as bacterial density rises. When their concentration crosses a predetermined threshold, they bind to receptor proteins and cause the expression of genes involved in the creation of biofilms [9]. This cellular communication can be broken down by QS inhibitors (QSIs) and quorum quenching (QQ) enzymes, which prevent the production of biofilms [10]. The use of QS-inhibiting drugs is a promising tactic for reducing bacterial infections because they can also increase bacterial sensitivity to antibiotics [11].
The lipopolysaccharide (LPS) carried by the Gram-negative bacterium Porphyromonas gingivalis (pg), which is frequently detected in the oral plaque of periodontitis patients and actively implicated in the progression of gingivitis, is recognised by Toll-like receptors (TLRs) on host cells [12, 13].
Many plants have antibacterial and anti-inflammatory qualities, and herbal rinses are used in traditional practises to maintain dental hygiene [12]. Scaling and root lanning (SRP), a common periodontal therapy [14], has been recommended as a supplement to a number of medications, including locally administered antibiotics [15, 16]. A perennial plant of European and Asian origin but now widespread in North America, the common comfrey (Symphytum officinale L., Boraginaceae) is well known for its anti-inflammatory effects and its capacity to speed up the healing of bruises, sprains, and wounds when applied topically [17]. Allantoin is a protein found in the roots and leaves of this plant that aids in bone and wound healing and cell proliferation [18, 19]. For ailments like colitis, rheumatoid arthritis, bronchitis, and gastric ulcers, comfrey is also used as a herbal tea. It is also a good source of protein and vitamin B12, two nutrients that are unusual for plants [20]. In this investigation, the oral pathogen P. gingivalis was tested for its antibacterial properties and its ability to suppress quorum sensing.
The results of this study may help in the search for new, all-natural antibacterial treatments and substances that can fight different dental disorders, particularly those that are active without altering the normal microflora of the oral cavity.
2. MATERIALS AND METHODS
2.1. Selection and Preparation of P. gingivalis Strains
Patients with periodontitis donated plaque samples. Gracey-curette injection made it possible to collect samples of subgingival plaque (Hu-Friedy, Chicago, USA). A subgingival plaque was gathered when the curette touched the bottom of the periodontal pocket without causing any harm to the soft tissues. The plaque sample was then submerged in sodium thioglycolate [12].
The process involved in obtaining the clinical isolates was conducted following the approval of the Local Ethical Committee (Ethical approval Reference number 382). All participants provided informed consent, and the study was performed in accordance with the Declaration of Helsinki.
2.2. P. gingivalis Strains were Carefully Selected for the Study based on their Relevance to Periodontal Diseases. The Following Steps were Undertaken
2.2.1. Identification and Selection of Suitable P. gingivalis Strains from Authenticated Culture Collections or Clinical Isolates
Twenty patients with periodontitis had their subgingival plaques collected. The samples were disseminated on BHI-blood Agar and then tested using P. gingivalis American Type Culture Collection, a standard strain (ATCC 33277), in sterilised screw-capped vials containing thioglycolate broth as a reducing transit medium for anaerobic bacteria, with 0.2 mg/ml of additional vitamin K and 10% blood (Sigma-Aldrich, St. Louis, MO). Each strain of the bacterium (ATCC 33277) and the patient's bacteria was cultivated for 48–72 hours at 37°C in an anaerobic chamber with 5% H2, 10% CO2, and 85% N2 [12].
2.2.2. Culturing and Maintenance of P. gingivalis Strains in Appropriate Growth Media under Anaerobic Conditions
In a brain-heart infusion medium with 10% blood and 0.2 mg/mL vitamin K, P. gingivalis was grown. (Sigma-Aldrich, Saint Louis, Missouri). An anaerobic chamber with 5% H2, 10% CO2, and 85% N2 at 37°C allowed bacteria to flourish for 48–72 hours [12].
For the two-fold serial dilution, ordinary saline was utilised. The following culture media were chosen for each microbial type and injected with them.
With the aid of a sterile microbiological spreader, 0.1ml of the inoculum was extracted and applied to the BHI agar plates. The plates were subjected to anaerobic incubation for 48 hours at 37 degrees Celsius using a gasPak delivered in an anaerobic jar.
2.2.3. Confirmation of P. gingivalis Identity Using Standard Microbiological Techniques, such as Gram Staining, Biochemical Tests, and Specific Molecular Markers
Gram stain was used to examine the appearance of isolated bacteria under a microscope. A glass slide was filled with a black-pigmented colony of microorganisms, mixed with a drop of DW, and allowed to dry before being heated to fix bacteria on the microscope slide. The slide was then washed with tap water before being stained with crystal violet for 1 minute to colourize the peptidoglycan cell and outer cell layers. After washing with tap water, the slide was stained with iodine for 1 minute, which produced significant crystal violet complexes in the outer cell layers. The enormous complex of its gram-negative bacteria was removed after decolorizing the slide with 95 percent ethanol for 20 seconds, and the slide was then cleaned with tap water. The slide was then counterstained for 40 seconds with safranine, washed with water, and allowed to dry. The slides were inspected under a light microscope to identify P. gingivalis after they had dried completely.
2.3. RapID ANA II System
In this study, the RapID ANA II system was used to evaluate the traits of the test organisms. Ten reaction cavities make up the system, and three to ten of them can accommodate two tests running simultaneously. The system produced results for 18 tests. The findings of the first and second tests, which assessed bifunctional tests both before and after the reagent's inclusion, were obtained, respectively. The bifunctional test cavities 3–9 that called for the RapID ANA II Reagent were identified by the first test being carried out above the bar and the second test being carried out below the bar. The RapID Spot Indole Reagent had to be used for Bifunctional Test 10, which was indicated by a box drawn around it.
The label lid covering the reaction chamber of the RapID ANA II panel was pulled off to start the testing procedure. The lower right-hand tab of the panel was pulled upward and to the left by firmly grasping it on the workbench (Fig. 1). Using the interpretation advice given in the table, cavities 1 (URE) through 10 (PO4) were read and scored without the use of any chemicals. Using the test code that was located above the bar, the test results for the bifunctional tests were typed into the relevant boxes on the report form.
In cavity 10, 2 drops of the RapID Spot Indole Reagent were used to carry out the particular bifunctional assays (IND). Additionally, cavities 3 (LGY) through 9 received 2 drops of the RapID ANA II Reagent (PYR). It was necessary to wait at least 30 seconds, but no longer than 2 minutes, after applying these reagents in order for the colour to develop. Following that, the samples were read and scored on a scale of three to 10. Using the test codes placed beneath the bar, the outcomes of the bifunctional testing were typed into the respective fields on the report form, as shown in Fig. (2).
The RapID ANA II system was employed in this study to assess the characteristics of the test organisms. The system consists of ten reaction cavities that generate 18 test results, with three to ten of these cavities capable of accommodating two different tests simultaneously. Bifunctional tests were evaluated twice, both before and after the addition of the reagent, yielding the first and second test results, respectively. The first test was conducted above the bar, while the second test was performed below the bar, indicating the bifunctional test cavities 3-9 that required the RapID ANA II Reagent. Bifunctional Test 10, on the other hand, necessitated the use of the RapID Spot Indole Reagent, which was denoted by a box drawn around it.
Angle of inoculation.
RapID ANA report form.
To begin the testing process, the label lid covering the reaction cavities of the RapID ANA II panel was peeled off. This was achieved by securely holding the panel on the workbench and pulling the lower right-hand tab upward and to the left. Without the addition of any chemicals, cavities 1 (URE) through 10 (PO4) were read and scored using the interpretation guidance provided in the table. For the bifunctional tests, the test results were entered into the appropriate boxes on the report form using the test code located above the bar.
To conduct the specific bifunctional tests, 2 drops of the RapID Spot Indole Reagent were added in cavity 10 (IND). Additionally, 2 drops of the RapID ANA II Reagent were applied to cavities 3 (LGY) through 9 (PYR). Following the application of these reagents, a waiting period of at least 30 seconds, but no more than 2 minutes, was allowed for color development. The samples were then read and graded based on a scale of three to ten. The results of the bifunctional testing were entered into the corresponding boxes on the report form using the test codes located beneath the bar.
2.4. MIC and MBC Determination
2.4.1. MIC and MBC Determination Protocol
A microbial inoculum was created by cultivating P. gingivalis strains in the proper culture media. Until the cultures reached the desired growth phase, they were incubated under ideal conditions. The exact estimate of inoculum density was made possible by the accurate measurement of bacterial concentration using spectro- photometry or colony-forming unit (CFU) counting.
By making successive dilutions of Metronidazole, Symphytum Officinale, and Panax Ginseng, the MIC was determined. Individual wells or tubes containing the P. gingivalis inoculum received various dosages of the test chemicals. The samples were then placed in an incubator with the right conditions to promote bacterial growth. The lowest dose of each test chemical that prevented discernible bacterial growth was determined by visually inspecting the plates or tubes after incubation. The MIC value was regarded as being at this concentration (Fig. 3).
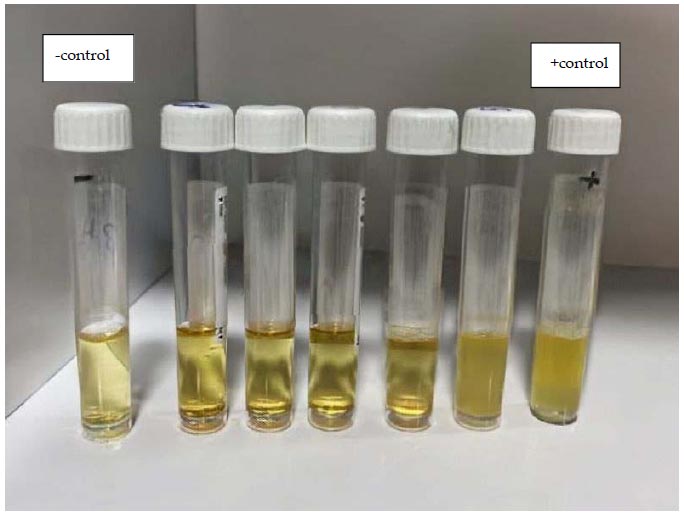
Evaluation of MIC, 5 tubes containing 2-fold serial dilution of (S,G,F) which were inoculated with 100 µl of P. gingivalis suspension, showed different growth of bacteria (turbidity).
Samples from the wells or tubes that showed no observable bacterial growth at the MIC concentration were subcultured onto solid agar plates for MBC measurement. The development of live bacteria was then permitted on these plates by incubation under the proper circumstances. The lowest concentration of each test chemical that caused no discernible bacterial growth on the agar plates was noted as the MBC value after the plates had been incubated.
To guarantee the accuracy and reproducibility of the results, each experiment was carried out in three duplicates. Each experiment used both positive and negative controls to ensure that the assay was accurate. Statistics were used to assess the significance of the differences between the test substances and the data from the MIC and MBC determination were recorded.
The antibacterial activity of Panax Ginseng, Symphytum Officinale, and Metronidazole against P. gingivalis was evaluated using the MIC and MBC determination procedure. The outcomes of this procedure gave vital insight into the optimum quantities of these compounds needed to stop bacterial development and get rid of the bacteria.
3. RESULTS
3.1. RapID ANA II
Based on the results obtained from the RapID ANA II panel, the following reactions and their corresponding values were observed:
1. URE: The test reaction value was 1, indicating a positive result.
2. BLTS: The test reaction value was 2, indicating a positive result.
3. aARA: The test reaction value was 4, indicating a negative result.
4. ONPG: The test reaction value was 1, indicating a positive result.
5. aGLU: The test reaction value was 2, indicating a negative result.
6. BGLU: The test reaction value was 4, indicating a negative result.
7. aGAL: The test reaction value was 1, indicating a negative result.
8. aFUC: The test reaction value was 2, indicating a negative result.
9. NAG: The test reaction value was 4, indicating a positive result.
10. PO4: The test reaction value was 1, indicating a positive result.
11. LGY: The test reaction value was 2, indicating a positive result.
12. GLY: The test reaction value was 4, indicating a positive result.
13. PRO: The test reaction value was 1, indicating a negative result.
14. PAL: The test reaction value was 2, indicating a positive result.
15. ARG: The test reaction value was 4, indicating a positive result.
16. SER: The test reaction value was 1, indicating a positive result.
17. PYR: The test reaction value was 2, indicating a positive result.
18. IND: The test reaction value was 4, indicating a positive result.
The test reactions URE and aARA both displayed a value of 1, signifying an unsuccessful outcome. A value of 2 was seen for the test reactions BLTS, ONPG, aGLU, aGAL, and aFUC, signifying a successful outcome. A value of 4 was also produced by the test responses BGLU, NAG, PO4, LGY, GLY, ARG, SER, PYR, and IND, indicating a significantly favourable outcome as shown in Table 1.
The outcomes list the reactions that were noticed together with the accompanying RapID ANA II panel values. These findings aid the identification and characterization of the examined microorganisms, which offer useful information on the precise reactions and their results, as shown in Figs. (4-6).
3.2. MIC and MBC Determination
The MIC results provide the lowest concentration of each test agent required to suppress audible bacterial growth. The MBC values are the test substance concentrations that are least likely to result in observable bacterial growth on agar plates. In comparison to the individual components (G, S, and F) alone, the results from strain ATCC33277 and bacteria collected from patients demonstrate considerable variance and a distinct influence of the combinations (G+F and S+F).
The Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of Panax ginseng (G), Symphytum Officinale (S), and Metronidazole (F) against those strains were determined using a strain of P. gingivalis (ATCC33277) and bacteria isolated from patients. Table 3 displays the results for bacteria taken from patients, while Table 2 lists the results for strain ATCC33277.
Table 2 shows the mean values of the MIC and MBC against P. gingivalis strain ATCC33277 for Panax Ginseng (G), Panax Ginseng with Metronidazole (G+F), Symphytum Officinale (S), Symphytum Officinale with Metronidazole (S+F), and Metronidazole (F).
| Test Reaction | Value | Results |
|---|---|---|
| URE | 1 | 0 |
| BLTS | 2 | 2 |
| aARA | 4 | 0 |
| ONPG | 1 | 2 |
| aGLU | 2 | 0 |
| BGLU | 4 | 0 |
| aGAL | 1 | 0 |
| aFUC | 2 | 0 |
| NAG | 4 | 99 |
| PO4 | 1 | 10 |
| LGY | 2 | 96 |
| GLY | 4 | 19 |
| PRO | 1 | 0 |
| PAL | 2 | 5 |
| ARG | 4 | 93 |
| SER | 1 | 27 |
| PYR | 2 | 27 |
| IND | 4 | 99 |
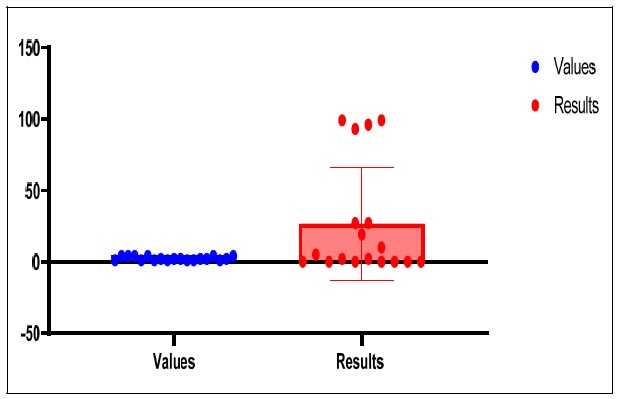
Comparison between values and results of RapID ANA II panel.
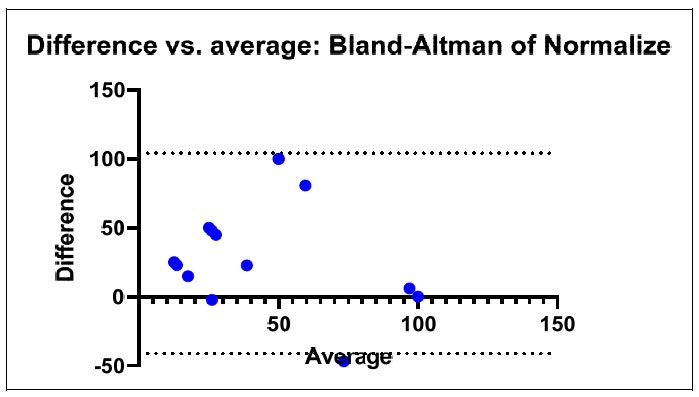
Differences and the average of the data extracted from the RapID ANA II panel.
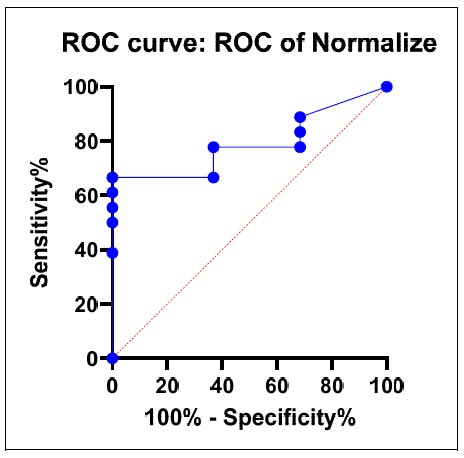
ROC curve.
| Strain | G | G+F | S | S+F | F |
|---|---|---|---|---|---|
| MIC | 250mg/ml | 1/4MIC G + 1/4MIC F | 82.5mg/ml | 1/4MIC S + 1/4MIC F | 125mg/ml |
| Patient | G | G+F | S | S+F | F |
|---|---|---|---|---|---|
| MIC | 250mg/ml | 1/4MIC G + 1/4MIC F | 82.5mg/ml | 1/4MIC S + 1/4MIC F | 250mg/ml |
Table 3 presents the mean values of the MIC and MBC against P. gingivalis isolated from patients with Panax Ginseng (G), Panax Ginseng with Metronidazole (G+F), Symphytum Officinale (S), Symphytum Officinale with Metronidazole (S+F), and Metronidazole (F).
The results show the average inhibition zone diameter (mm) for P. gingivalis strain ATCC 33277 and the isolate at various doses of Symphytum Officinale (S). As the concentration of Symphytum Officinale dropped, the strain ATCC 33277 displayed shrinking inhibitory zone diameters. When Symphytum Officinale was present in lesser concentrations, the isolate similarly displayed similar tendencies, with shrinking inhibitory zone widths. When the significance level was set to P <0.01, the statistical analysis, as shown by the X2 and P-value, revealed highly significant differences (H.S.).
4. DISCUSSION
In this study, we examined the effectiveness of Metronidazole, Symphytum Officinale, and Panax Ginseng as antibacterial agents against P. gingivalis [21]. The findings of the MIC and MBC analysis and the RapID ANA II panel testing provide important information about how effective these compounds are against P. gingivalis [22].
The results of the MIC and MBC tests showed that the combinations (G+F and S+F) had distinct effects and substantial differences from the individual compounds (G, S, and F) alone. Lower MIC and MBC values were seen in the Panax Ginseng and Metronidazole (G+F) and Symphytum Officinale and Metronidazole (S+F) combinations compared to the individual drugs alone, demonstrating a synergistic effect [23]. These results imply that combination treatment may increase the antibacterial activity against P. gingivalis and may boost therapeutic results.
Thefindings of the RapID ANA II panel shed light on the particular test reactions and their results [23]. Certain enzymes or metabolic processes are necessary for the positive reactions of BLTS, ONPG, NAG, PO4, PAL, PYR, and IND [24]. The negative responses seen for URE, aARA, aGLU, BGLU, aGAL, aFUC, PRO, and SER suggest the absence of related enzymes or metabolic processes. These findings can help with the identification and characterization of the examined microorganisms because they are consistent with the expectations of the RapID ANA II panel for the reactions [25]. Additionally, the results of Symphytum Officinale for inhibitory zone diameter against ATCC 33277 and isolated strains of P. gingivalis showed a concentration-dependent effect [26].
The mean inhibitory zone diameter decreased along with the reduction in Symphytum Officinale concentration [12, 27]. This shows that Symphytum Officinale may have better antibacterial activity against P. gingivalis at higher doses. However, more research is required to find the ideal concentration for effectively inhibiting P. gingivalis growth [28]. Overall, the findings of this study demonstrate the potential antibacterial efficacy of Metronidazole, Symphytum Officinale, and Panax Ginseng against P. gingivalis.
In comparison to single drugs, combination therapy (G+F and S+F) showed increased effectiveness. The RapID ANA II panel also offered helpful data for the characterisation and identification of the tested microorganisms [29]. These findings aid in the creation of alternative P. gingivalis infection treatments, particularly in situations where traditional antibiotics may not be successful [30].
CONCLUSION
The findings of this study highlight the potential antibacterial effects of Panax ginseng and Symphytum officinale in conjunction with metronidazole against P. gingivalis. According to the results of the MIC and MBC analysis, the combination therapies (G+F and S+F) display synergistic effects and have lower MIC and MBC values than the individual drugs alone. This suggests that the ability of Metronidazole to treat P. gingivalis infections may be improved by the combination of Panax ginseng and Symphytum Officinale.
LIST OF ABBREVIATIONS
| MIC | = Minimum Inhibitory Concentration |
| MBC | = Minimum Bactericidal Concentration |
| CFU | = Colony-forming Unit |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study involving clinical isolates was conducted following the approval of the Local Ethical Committee (Reference number 382), the College of Dentistry at the University of Baghdad granted the authorization for the protocol of 12 of 15 studies.
HUMAN AND ANIMAL RIGHTS
All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committee and with the 1975 Declaration of Helsinki, as revised in 2013.
AVAILABILITY OF DATA AND MATERIALS
Upon a reasonable request, the data created and examined during the current study can be made available.


