All published articles of this journal are available on ScienceDirect.
An in vitro Evaluation of pH Variation and calcium ion release of three Endodontic Sealing Cements: BioRoot RCS, MTA Fillapex, and Acroseal
Abstract
Introduction:
For several years, calcium silicates have proven to be very useful in numerous endodontic or restorative clinical situations. Derived from Portland cement and later from Mineral Trioxide Aggregate (MTA), a new generation of calcium silicate-based cement is marketed.
To meet the requirements of endodontic sealing cements, several modifications according to the original formulation of calcium silicate and several additives have been implemented to create materials with excellent physical properties and endowed with the biological properties of MTA.
Aim:
The objective of the present in vitro study was to evaluate the variation in pH and the release of calcium ions in three endodontic cements over a period of time ranging from 3 hours to 28 days. The evaluation involved the following cements: BioRooT RCS (a pure calcium silicate-based endodontic cement), MTA Fillapex (a Mineral Trioxide Aggregate-based endodontic cement), and Acroseal (a calcium hydroxide-based endodontic cement).
Materials and Methods:
In this in vitro study, three root canal sealant cements were compared. A total of fifty four samples were prepared, and they were divided into three groups: Group 1 included 18 samples of BioRoot RCS, group 2 involved 18 samples of MTA Fillapex, and Group 3 included 18 samples of Acroseal. The samples were prepared and inserted into the molds. Then, the molds were immersed in glass test tubes containing 10 ml of double-distilled deionized water. A control tube, containing no material, was also prepared for each group. After each experimental period, the samples were taken from the tubes using sterile tweezers and weighed after 3 hours, 24 hours, 48 hours, 7 days, 14 days, and 28 days, respectively. The liquid in which the samples were previously immersed was used for measuring pH and the release of Ca ++ ions. Measurements of pH were carried out with a pH meter, previously calibrated using a buffer solution (pH 7). Measurement of the release of calcium ions was carried out using the technique of complexation of calcium ions with ethylene-diamine-tetra-acetic-acid, better known by the acronym EDTA. Statistical analysis was performed using data processing software, SPSS Statistics v.21.0. In this analytical study, two statistical tests were used for data analysis: A Two-factor ANOVA test and a linear regression test for comparison of quantitative variables.
Results:
The results of the present study showed that compared to other materials, BioRoot RCS had the greatest calcium ion release. An ion release that is prolonged over time and which remains markedly high. The analytical study showed that BioRoot RCS had higher pH and calcium ion release values than the other two materials. These values are statistically significant (p<0.05) with a strong correlation between the release of calcium ions and the variation in pH.
Conclusion:
BioRoot RCS, a cement based on pure calcium silicate, showed an alkalinizing activity and an ion release power clearly superior to those of sealers based on MTA and calcium hydroxide. This is largely correlated with the chemical composition and the physicochemical behavior of the material.
1. INTRODUCTION
For several years, calcium silicates have proven to be very useful in numerous endodontic or restorative clinical situations. Derived from Portland cement and following on Mineral Trioxide Aggregate (MTA), a new generation of calcium silicate-based endodontic cement is marketed [1, 2].
Endodontic sealers must meet the criteria well established by Grossman: easy to handle, obturate the entire canal system including ramifications, having no grip retraction, being insoluble in fluids, being antibacterial or at least unfavorable for bacterial proliferation, having sufficient radiopacity, not causing discoloration of dental structures, being non-irritant for peri-apical structures and easy to remove, if necessary.
To meet the requirements of endodontic sealing cements, several modifications according to the original formulation of calcium silicate and several additives have been implemented to create materials with excellent physical properties, and endowed with the biological properties of MTA [3, 4].
These biological properties are essentially the result of the sealer’s ability to maintain an alkaline pH responsible for antibacterial action and to release calcium ions playing a key role in wound healing and bio-mineralization. The role of hydroxyl and calcium ions has been well-established since the first studies performed on calcium hydroxide [2].
We propose, in this study to compare the performances of three root canal sealant cements: BioRoot RCS (Septodont, Saint-Maur-des Fosses, France), MTA Fillapex (Angelys, Brasil), and Acroseal (Septodont, Saint-Maur-des Fosses, France) through an experimental study evaluating the pH and the release of calcium ions responsible for antimicrobial biological activities.
2. MATERIALS AND METHODS
2.1. Materials
The three root canal sealant cements compared in this in vitro study are presented in the following tables (Tables 1 and 2).
Other materials were used in this experimental study: Sterile glass plates, mixing spatulas, sterile tweezers, plastic molds, sterile plastic tubes, sterile compresses, Stirrer a stirrer, oven set to 37 ° C, precision balance to 1 / 1000th g, pH-meter that allowed to measure the pH of the solution; titration by complexation of calcium with EDTA Erlenmeyer flask, micropipette, graduated pipette, titrated EDTA solution, color indicator from Patton and Reeder, and potassium cyanide solution.
2.2. Methods
2.2.1. Preparation and Decontamination of Molds
In order to have samples of standard shape and volume, plastic molds of cylindrical shape, with a height of 5mm and an internal diameter of 4mm or a volume equal to 62.83 mm3, were prepared. A total of 54 molds were prepared, including 18 molds for each material and a sample number of n = 3 for each evaluation interval. The mussels were decontaminated by immersion in ethanol for 20 minutes.
| Materials | Firm | Presentation | Lot Number |
|---|---|---|---|
| BioRoot RCS | Septodont, Saint-Maur-des Fosses, France | liquid/powder | B191453 |
| MTA Fillapex | Angelys, Brasil | Double syringe with mixing tip | 44680 |
| Acroseal | Septodont, Saint-Maur-des Fosses, France | two catalyst base pastes | B20128 |
| Materials | Bioactive Cement | Composition |
|---|---|---|
| BioRoot RCS | Tricalcium Silicate | • Powder: Tricalcium Silicate, povidone, zirconium oxide • Liquid: calcium chloride, water soluble polymer |
| MTA Fillapex | Mineral Trioxide Aggregate |
• Base Mineral Trioxid Aggregate, Base resin, Titanium dioxide: pigment • Catalyst: Salicylate resin, Bismuth oxide, filler |
| Acroseal | Calcium hydroxide | • Base: bismuth carbonate, esterified rosin, TCD-diamine, Aerosil (colloidal silica) • Catalyst: bismuth carbonate, calcium hydroxide, diglycidyl ether bisphenol A, enoxolone. |
2.2.2. Preparation of Samples
The three materials were prepared according to the manufacturer’s instructions and were inserted into the molds intended for this purpose. A total of 54 samples were prepared and they were divided into three groups:
• Group 1 included 18 samples of BioRoot RCS (a sample number of n = 3 for each evaluation interval)
• Group 2 included 18 samples of MTA Fillapex (a sample number of n = 3 for each evaluation interval)
• Group 3 included 18 samples of Acroseal (a sample number of n = 3 for each evaluation interval)
2.2.3. Weight Measurement
For the purpose of standardization of measurements, the plastic molds were weighed empty, and then they were weighed after filling to make sure the same amount of material was put for all the samples.
2.2.4. Dissolution of Samples
The samples were prepared and inserted into the molds. Then, the molds were immersed in glass test tubes containing 10 ml of double-distilled deionized water. The tubes were labeled and distributed as follows:
• 18 tubes for BioRoot RCS (6x3)
• 18 tubes for MTA Fillapex (6x3)
• 18 tubes for Acroseal (6x3)
A control tube, containing no material, was also prepared for each group. The pH of double-distilled water was measured before immersion of the samples in order to verify its neutrality (pH = 7).
2.2.5. Weight Measurement during the Experiment
After each experimental period, the samples were taken from the tubes using sterile tweezers and they were weighed after 3 hours, 24 hours, 48 hours, 7 days, 14 days, and 28 days, respectively. The liquid in which the samples were previously immersed was used for measuring the pH and the release of Ca ++ ions.
2.2.6. PH Measurement
Measurements of pH were carried out with a pHmeter previously calibrated using a buffer solution (pH 7). The ambient temperature during the measurements was automatically specified according to the pH meter used.
Each tube was placed in a TOP-mix 94223 (Heidolph) vibrator for 5 seconds in order to homogenize the solution before measuring the pH. Immediately after calibrating the pH meter and stirring the solutions, pH measurements were started. Three measurements were made for each time interval and for each material (3h, 24h, 48h, 7 days, 14 days, and 48 days). The pH measurement was performed by inserting the pH meter electrode into the tube and then reading the value displayed on the screen after stabilization. The pH of double-distilled and deionized water in the control tube was measured at all time intervals to ensure its neutrality.
2.2.7. Measurement of the Release of Calcium Ions
Measurement of the release of calcium ions was carried out using the technique of complexation of calcium ions with ethylene-diamine-tetra-acetic acid, better known by the acronym EDTA. The standard solution of EDTA at the known concentration (2.5 mMol / L) was introduced in a pipette. In an Erlenmeyer, a volume of 1 ml of the studied samples, 5 ml of double-distilled water, a pinch of Patton and Reeder color indicator, and 0.5 ml of sodium cyanide solution were introduced.
The introduced colored indicator had a pink coloring when it was introduced into the solution. When all the calcium ions present in the solution were complex, its color turned blue. EDTA complexation was not specific to only calcium ions, it exhibited an affinity to magnesium ions. It was therefore necessary to complex the magnesium ions in a basic medium: Mg2 + + 2 OH- = Mg (OH) 2. Likewise, the characteristics of the colored indicator required work in a basic environment.
To ensure its conditions, sodium cyanide solution was added to the mixture. For the complexation of calcium ions, EDTA was added dropwise to the mixture in the Erlenmeyer flask while mixing. When the color changed from pink to blue, the volume of the added EDTA was noted. Calculation of calcium ions concentration in the sample was done according to the following formula:
[Calcium] x V (Calcium) = [EDTA] x V (EDTA)
Given that,
• The volume of Calcium = 1 ml
• [EDTA] = 2.5 mMol / L
The equation became:[Calcium] in mMol / L = 2.5 x V
2.2.8. Statistical Analysis
Statistical analysis was performed using data processing software: SPSS Statistics v.21.0. In this analytical study, two statistical tests were used for data analysis:
• ANOVA test for the comparison of five quantitative variables (material, pH, release of calcium ions, weight, and time) of independent samples.
• Linear regression analysis test for the comparison of five quantitative variables.
Thus, five variables were defined during data analysis:
• Material: BioRoot RCS, MTA Fillapex, Acroseal
• The pH of each material during all the study periods.
• Ca ++: the release of calcium ions during all the study periods.
• Weight: the weight of the samples before and during the study periods.
• Time: 0h, 3h, 24h, 48h, 7 days, 14 days, and 28 days.
3. RESULTS
3.1. Comparative Analysis of pH Values
ANOVA statistical results of the pH values of the three materials are shown in Table 3.
This table represents the averages of the pH measurements at each interval, as well as the standard deviation between the various tests carried out for each material (n = 3).
The materials were compared in pairs at each measurement time to determine if there was a significant difference between the pH values. A significant difference was noted between the three materials at all measurement times (P <0.05), with the exception of the pH value at 3h between MTA Fillapex and Acroseal, where no significant difference was noted (P> 0.05).
Table 3.
| P (PH) | 3H | 24H | 48H | 7d | 14d | 28j |
|---|---|---|---|---|---|---|
| BioRoot /MTA Fillapex | 0,000004030 | 0,000047067 | 0,000113390 | 0,000053090 | 0,000019927 | 0,000133507 |
| BioRoot/ Acroseal | 0,011396402 | 0,000005641 | 0,000029131 | 0,000014546 | 0,000000021 | 0,000023012 |
| MTA Fillapex/Acroseal | 0,277423483 | 0,000027961 | 0,000071531 | 0,000013302 | 0,000220717 | 0,042381988 |
3.2. Linear Regression Analysis
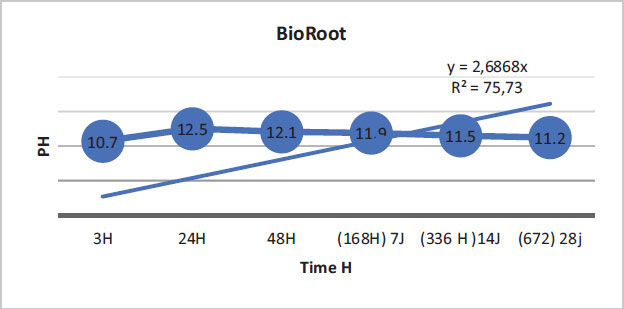
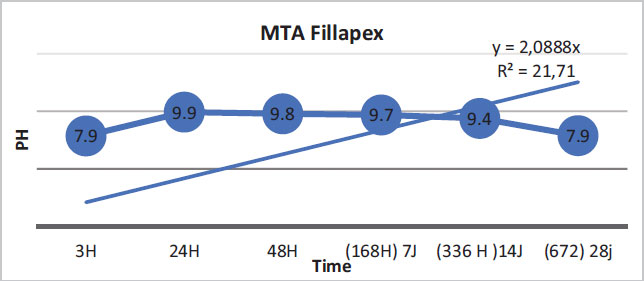
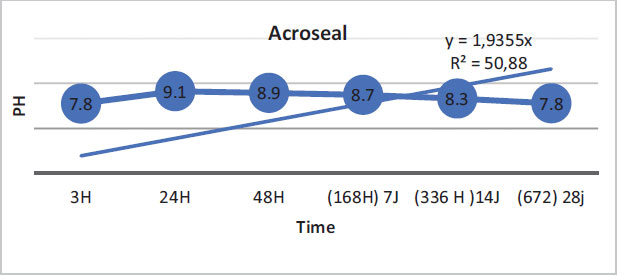
The regression curves of the pH values for the three materials are illustrated by the following figures (Figs. 1-3).
BioRoot showed that 75.73% of the variation in pH values was explained by time. The link between time and pH variation was demonstrated. (Fig. 1).
For Fillapex MTA, only 21.71% of the variation in pH values was explained by time. The link between these two variables was weak. However, this may also be due to the inadequacy of the simple linear regression model in showing the variation in pH values (Fig. 2).
For Acroseal, 50.88% of the variation in pH values was explained by time. This finding showed that the correlation link was average (Fig. 3).
| P | 3H | 24H | 48H | 7d | 14d | 28d |
|---|---|---|---|---|---|---|
|
BioRoot / MTA Fillapex |
0,000134885 | 0,000029965 | 0,000715297 | 0,000058679 | 0,000000240 | 0,000174257 |
|
BioRoot/ Acroseal |
0,000002980 | 0,000005000 | 0,000000163 | 0,000007684 | 0,000000162 | 0,000162765 |
|
MTA Fillapex/ Acroseal |
0,016149255 | 0,004335831 | 0,000338685 | 0,010309633 | 0,001257716 | 0,002852768 |
3.3. Calcium Ions Release Analysis
3.3.1. Comparative Analysis of Calcium Ions Release Values
ANOVA statistical results of calcium ions release values of the three materials are shown in Table 4. This table represents the mean calcium ions release measurements at each interval, as well as the standard deviation between the different tests carried out for each material (n = 3).
The materials were compared in pairs at each measurement time to determine if there was a significant difference between calcium ion release values. A significant difference (p <0.05) was noted between the three materials at all measurement intervals.
3.4. Linear Regression Analysis
For BioRoot, only 15.86% of the variation in pH values was explained by time.
For Fillapex MTA, only 1.46% of the variation in pH values was explained by time.
For Acroseal, only 2.18% of the change/variation in pH values was explained by time.
Therefore, the link between these two variables was weak. However, this may also be due to the inadequacy of the simple linear regression model to show/in showing the variation in pH values.
3.4.1. Weight Analysis
The mean values of weight variation are shown in Table 5.
Variations after statistical analysis are shown in Table 6. The materials were compared in pairs.
The variation in weight between BioRoot and Acroseal was significant (P <0.05) however, the variation between BioRoot and MTA Fillapex as well as the variation between MTA Fillapex and Acroseal were not significant (P> 0.05).
| Weight mg | BIOROOT | MTA | ACROSEAL |
|---|---|---|---|
| 0h | 154 | 156 | 155 |
| 3H | 150 | 152 | 153 |
| 24H | 146 | 150 | 151 |
| 48H | 143 | 149 | 150 |
| 7d | 142 | 148 | 149 |
| 14d | 141 | 146 | 147 |
| 28d | 140 | 144 | 146 |
| - | BIOROOT/MTA Fillapex | BIOROOT/ACROSEAL | MTA Fillapex/ACROSEAL |
|---|---|---|---|
| P | 0,06 | 0,03 | 0,3 |
| - | BioRoot | MTA Fillapex | Acroseal |
|---|---|---|---|
| Correlation Coefficient | 0,83 | 0,72 | 0,73 |
| - | P | |||||
|---|---|---|---|---|---|---|
| BIOROOT | 0,004 | - | - | - | - | - |
| Calcium | 9,27 | 11,16 | 10,41 | 9,75 | 8,83 | 7,33 |
| PH | 10,71 | 12,53 | 12,05 | 11,91 | 11,52 | 11,22 |
| MTA FILLAPEX | 0,001 | - | - | - | - | - |
| Calcium | 0,4 | 4 | 5,25 | 1,96 | 1,66 | 0,87 |
| PH | 7,87 | 9,92 | 9,79 | 9,68 | 9,39 | 7,89 |
| ACROSEAL | 0,001 | - | - | - | - | - |
| Calcium | 0,91 | 1,33 | 1,83 | 1,58 | 0,91 | 0,57 |
| PH | 7,75 | 9,13 | 8,94 | 8,72 | 8,33 | 7,79 |
3.5. Study of the Correlation Between the Release of Calcium Ions and pH
The correlation coefficient explains the relationship between the variation in pH and the release of calcium ions. If the correlation coefficient is positive and practically equal to 1 and the curve is linear, there is a relationship between the two variables. If the correlation coefficient is negative, there is no relationship between the variables.
The correlation coefficient between pH variation and calcium release of the three tested materials is shown in (Table 7).
As shown in this table, the correlation coefficient values for the three materials are close to 1, which may indicate a relationship between the variation in pH and the change in calcium release. However, these coefficients must be compared with the results of linear regression analysis to confirm this relationship.
The statistical analysis of the correlation between the release of calcium ions and the variations in pH values is shown in the following Table 8.
According to the statistical analysis, there was a significant variation in pH values depending on the release of calcium ions for the three materials (P <0.05).
4. DISCUSSION
Endodontic therapy has generously benefited from materials based on calcium silicate, commonly referred to as bioceramics or MTA-based. These hydrophilic materials have good biocompatibility and are able to form hydroxyapatite crystals. The use of bioceramic sealers has a considerable number of advantages for endodontic therapy [1, 2].
• They are highly hydrophilic; so, the natural moisture of the canal and tubules is an advantage.
• When curing, the sealer does not contract, unlike other types of sealers, but it exhibits a slight expansion improving the sealer's tightness.
• They have a strongly alkaline pH, ensuring antibacterial activity.
• They are biocompatible and they promote bio-mineralization and the formation of hydroxyapatite deposits, a determining factor for the healing of periapical lesions.
• The use of gutta cones covered with bioceramic nanoparticles ensures a bond between the sealer and the gutta for a better seal.
In order to modify and adapt their physical properties, several modifications to their formulations were made and different agents were added. These modifications have notably improved the physical properties but they can also interfere with the chemical and biological properties of these materials. So, it seems to be a very good idea to study their physicochemical properties [3, 4]
The aim of the present study was to evaluate the pH variation and the release of calcium ions in three endodontic sealing cements, which are: BioRoot RCS, MTA Fillapex, and Acroseal.
In this study, BioRoot RCS was found to have a very high pH compared to MTA Fillapex and Acroseal. Moreover, BioRoot RCS had a greater calcium ion release capacity than the other two endodontic sealers. At the beginning of this experiment, after mixing and immediately placing the aqueous solution in deionized water, it was noted that the pH of BioRoot, equal to 10.71 at 3 h, was higher than those of MTA Fillapex and Acroseal, which were equal to 7.86 and 7.75, respectively. Up to 24 h, the pH values of the three materials increased over time. The pH values of BioRoot remained significantly higher than those of the other materials. They were 12.53 for BioRoot, 9.92 for MTA Fillapex, and 9.12 for Acroseal. These measurements were the maximum values over the entire test period for the three materials. At 48 hours, the values started to decrease slowly for the three materials, but they remained significantly higher for BioRoot compared to the other two materials.
The present study showed that BioRoot RCS had the highest pH values compared to the other two materials. The alkalinization capacity of BioRoot persisted throughout the test period despite the decrease in pH values over time, and they remained significantly higher than the other two materials. It is important to note that MTA Fillapex showed higher pH values than Acroseal over the entire study period.
In the present study, BioRoot RCS had a significantly greater calcium ion release capacity compared to the other two materials. This release was extended throughout the test period for BioRoot RCS and MTA Fillapex, but it was higher for BioRoot. Acroseal showed the lowest concentrations of calcium ions release.
The concentrations reported in the present work were of the order of Mmol / L.
At the first 3-hour measurement, a significant release of calcium ions was noted for BioRoot RCS. The concentration was 9.27. It was 1 for MTA Fillapex and 0.9 for Acroseal. At 24-hour measurement, the values increased markedly for BioRoot RCS and MTA Fillapex. They were 11.15 and 4, respectively. The concentration for Acroseal was 1.32. At this stage of measurement, this value of 11.15 was the maximum concentration for BioRoot RCS over the entire test period.
At 48 h/the 48-hour measurement, a decrease in the release of calcium ions was noted for BioRoot RCS. The concentration decreased from 11.15 to 10.4. In contrast, the concentrations increased for MTA Fillapex (5.25) and Acroseal (1.82). These values were the maximum concentrations for these two materials. But/Yet, the concentrations remained much higher for BioRoot RCS. After 7 days, calcium ion concentrations decreased for all the materials to reach 9.75, 1.95, and 1.57 for BioRoot RCS, MTA Fillapex, and Acroseal, respectively. A significant decrease in the release of calcium ions was noted for MTA Fillapex.
On the 28th day, a gradual decrease was noted in ion concentrations, but the values remain/remained higher for BioRoot RCS.
BioRoot RCS exhibited the greatest calcium ion release compared to the other materials, an ion release that was prolonged over time and which remained markedly high.
The analytical study showed that BioRoot RCS had higher pH and calcium ions release values than the other two materials. These values were statistically significant. In addition, a strong correlation was found between the release of calcium ions and the variation in pH.
These results are consistent with those found in several studies. The study carried out by Siboni et al. in 2017 [5] compared the variation in pH and the release of calcium ions in three materials, namely MTA Fillapex, BioRoot RCS, and AH Plus over a 28-day period, using time intervals which were: 3h, 24h, 72h, 7 days, 14 days, and 28 days. In the aforementioned study, the prepared samples were immediately immersed in a solution of 10 ml of deionized water. To measure the release of calcium ions, the authors used the technique of atomic absorption spectrophotometer. In this study, they reported that BioRoot RCS significantly increased the pH of water after submersion compared to the other materials for the first 14 days. The pH of BioRoot RCS was around 11-12 for the first 14 days, with a maximum value of 12.1 at 24 hrs. Then, after 28 days, the pH dropped to 8.7, but alkalization was still present. During the first 7 days, MTA Fillapex exhibited an alkaline pH varying between 9.1 and 9.5, and the maximum value of 9.5 was observed between 3 hours and 24 hours. Then, the pH decreased significantly after the 7th day to become practically neutral at 28 days/day 28 (pH = 7.1). The authors reported that the alkalizing activity was significantly higher for BioRoot RCS, especially during the first 14 days. In the same study, for BioRoot RCS, the release of calcium ions started immediately after immersion. It was reported to be in the order of 721.4 ppm at 3 hrs. Then, it gradually decreased throughout the experimental period, but it remained significantly elevated to 28 days (40.4 ppm). For MTA Fillapex, the maximum release (31 ppm) was noted to be between 1 and 3 days. Then, it decreased to 15.9 ppm at 28 days/day 28. In this study, the authors also evaluated the deposition of hydroxyapatite crystals on the surface of the materials using a scanning electron microscope. The thickest layer of deposit was observed on the surface of BioRoot RCS. According to the authors, the result was in agreement with the cumulative calcium release data after 28 days of immersion.
An in vitro study was carried out by Urban et al. in 2018 in order to compare three endodontic sealers, namely BioRoot RCS, MTA Fillapex, and AH Plus, and to evaluate the pH variations of the 3 materials over the long term (6 months) [6]. The materials were tested after setting. The measurements started 48 hours after mixing. The pH values were estimated at 6 time intervals: 12h, 14 days, 1 month, 2 months, 4 months, and 6 months. BioRoot was reported to have the highest pH values compared to the other materials. The maximum pH value was recorded at 12 h for the three materials: BioRoot RCS (12.1), MTA Fillapex (10.3), and AH Plus (9.3). Then, the pH values decreased throughout the test period for the 3 materials, but with significantly higher values for BioRoot compared to the other materials. At 1 month, the values were: 11.7, 10.3, and 8.2 for BioRoot RCS, MTA Fillapex, and AH Plus, respectively. At 6 months, the values decreased to reach 10.3, 8.8, and 6.3 for BioRoot, MTA Fillapex, and AH plus, respectively. The authors reported that maintaining an alkaline environment over a fairly long period (6 months) can play a positive role in the healing of the lesion in the periapical region.
In 2016, Khalil et al. [7] evaluated the variation in pH and the release of calcium ions in two sealers based on tricalcium silicate, namely BioRoot RCS and BIO MM. The materials were tested after mixing and immediate immersion in Hank's balanced solution. For the measurement of the release of calcium ions, the method of inductively coupled plasma spectrometry was used. BioRoot presented the most important pH values: 12.1 to 24 hours and 12.7 to 28 days in comparison with the BIO MM: 10.9 to 24 hours and 11.9 to 28 days. However, they were 8.4 to 24 hours and 8.7 to 28 days for AH Plus. It is important to note that the pH values increased during the test period from 1 day to 28 days in the aforementioned study, which is inconsistent with the results reported in the present study as well as those reported in the literature. This may be due to the immersion medium, which was not distilled water but Hank's Balanced Solution (HBSS). Likewise, BioRoot was reported to have the highest concentrations of calcium ions. The estimates were 19789 mg/g at 24h and 28682 mg/g at 28 days, in comparison with 7200 mg/g at 24h and 2333 mg/g at 28 days for BIO MM.
In 2007, Eldeniz et al. [8] compared the variations in pH and the release of calcium ions in three calcium hydroxide-based sealers, namely Acroseal, Apexit, and Sealapex. The materials were evaluated after immediate immersion in distilled water, before setting. The pH values for Acroseal were 7.4, 7.6, and 7.8 at 24 hours, 14 days, and 28 days, respectively. The concentrations of the calcium ions released were 0.5 mg/dl at 24 h, 0.9 mg/dl at 14 days, and 1.2 mg/dl at 28 days. In this aforementioned study, the estimated values for Acroseal were the lowest among the three materials studied, which is in agreement with our present study.
However, unlike the results reported in the present study, the pH and calcium ion values increased during the test period. The results found by Eldeniz (2007) were confirmed by another study carried out by Verma et al. (2014) [9]. In this study, in which the pH and calcium ion release values decreased over time, is consistent with the results of the present study.
The authors reported that the relatively low hydroxyl and calcium ion release values for Acroseal are explained by its very low solubility as it exhibited an epoxy resin matrix containing ethyldiglycidyl bisphenol A and methenamine. The same results of low ionic release and very low ability to induce biomineralization were confirmed in the study conducted by Bueno et al. (2016) in a study where the authors compared the biocompatibility and biomineralization of Acroseal, MTA Fillapex, and Sealapex. Giuroiu et al. (2018) [10] compared the pH variations of four endodontic sealers, namely MTA Fillapex, Acroseal, EndoFlas, and Endomethasone over a period of 21 days. The pH value of Acroseal was 8.5 at 24h, and it increased to 8.73 at 48h. The difference in pH value in comparison with the present study may be due to the fact that Acroseal was evaluated after complete setting. The pH value returned to 8.5 at 7 days and it showed a further increase and reached 8.79 at 21 days. The pH of MTA Fillpex was 9.23 at 24 hours and it increased to 9.70 at 48 hrs. After 7 days, the pH rose to 9.83 and it exceeded 10.16 on day 14, followed by a slight drop to 9.99 on day 21.
De Prullage et al. (2016) [11] compared the solubility of three endodontic sealers, namely BioRootRCS, MTA Fillapex, and AH Plus. In this study, BioRoot showed/was reported to show the highest solubility compared to the other two materials. The authors reported the formation of calcium hydroxide early in the setting process for BioRoot RCS, which was not the case with MTA Fillapex. Thus, BioRoot RCS probably promoted the greater release of OH- and Ca 2+ ions. For calcium silicate cement, the materials with higher solubility were reported to have higher OH- and Ca 2+ content. Several studies reported results that are consistent with this study, confirming the greater solubility of BioRoot compared to other sealers, more particularly MTA Fillapex [12, 13, 5]. Solubility is therefore important for the release of calcium and hydroxyl ions to ensure their biological and antimicrobial effects. This solubility can suggest that it decreases their sealing capacity over time and can lead to micro-leaks [5]. However, this loss of seal is considered minimal and does not affect the seal of calcium silicate materials. Several authors evaluated the tightness of root canal fillings with bioceramic sealers. These sealers had shown excellent sealing ability, which can be explained by several phenomena taking place during the material setting reaction: the expansion capacity after setting, the ability to form micro-mechanical adhesion with the root canal dentin by penetration of Hydroxyapatie microparticles in the dentinal tubules at the tooth -material interface, ensuring the sealing of this interface. [14-17].
The values found in the present study are in agreement with those of other studies concerning the strong release of hydroxyl and calcium ions, explaining the biocompatibility and bioactivity of BioRoot RCS. Sustained release of calcium ions had been shown to be a key factor in promoting the pulp and in periapical regeneration [18]. The sustained release capacity of calcium ions and the formation of hydroxyapatite deposits may explain the excellent biocompatibility and bioactive potential, reported in vitro, of BioRoot when contacted with stem cells [5]. Camps et al. (2015) [19] showed that BioRoot RCS placed in contact with periodontal ligament cells stimulated in vitro the production of angiogenic and osteogenic growth factors.
In another study conducted by Dimitrova-Nakov et al. (2015) [20], evaluating the bioactivity of BioRoot, the authors showed that BioRoot maintained the potential for cell regeneration and can induce mineralization by stimulation of the BSP-DMP 1 factor.
Tricalcium silicate, the main component of BioRoot RCS, has been proven to increase cell proliferation and promote osteogenic differentiation. The release of calcium promotes cell proliferation and differentiation. BioRoot RCS released Ca 2+ and OH- and created an alkaline environment.
Ca 2+ release and alkalinizing activity were significantly higher and more prolonged in BioRoot RCS than in MTA-Fillapex. This could be due to the high solubility of BioRoot RCS and it could be responsible for its superior cytocompatibility [21]. In addition, the main component of MTA-Fillapex was a salicylate resin matrix, while MTA was a minor additive. MTA-Fillapex, therefore, absorbs less water, releases much less Ca 2+, and exhibits less alkalizing activity and fewer apatite deposits than calcium silicate cement. This might be correlated with the hypothesis that the salicylate resin matrix made MTA relatively inert [7, 22].
CONCLUSION
BioRoot RCS, cement based on pure calcium silicate, showed an alkalinizing activity and a calcium ion release power clearly superior to those of the sealers based on MTA and calcium hydroxide. This is largely correlated with the chemical composition and the physicochemical behavior of the material.
In fact,
• The difference in hydration between pure calcium silicate and Portland cement is contained in MTA, interferes with the formation and dissociation of calcium hydroxide.
• The existence of elements such as bismuth oxide and silicone oxide in the composition of MTA, also interferes with this hydration reaction.
• The resinous matrix, present in a large percentage in the composition of MTA Fillapex and Acroseal, acquires an inert behavior that limits the release of ions.
• Finally, the solubility property is also correlated with the importance of the ionic release of the material.
Thus, Endodontic treatments will eventually have to turn to the use of increasingly “purified” silicate cement (without resinous components), such as zirconium oxide that is present in BioRoot RCS (Septodont). Nevertheless, several other specific points still remain to be studied, in particular their mutagenic action and their long-term efficacy. The effect of large ionic release on the sealing of the root canal filling is to be investigated further.
More clinical investigations will therefore be necessary for these new biomaterials to be modified and developed in order to overcome the few remaining challenges in the search for the ideal cement.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used in this research.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data and supportive information are available within the article.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


