All published articles of this journal are available on ScienceDirect.
Dental Tissue Engineering by Neural Differentiation of Dental Stem Cells and Nano-systems: A Review
Abstract
Background:
Pulpitis is a pulpal inflammation. It generally occurs when there is inflammation within a tooth as a result of anything like grinding or decay. After dental inflammation, microcirculation and sensory nerve activity seem to play the most critical role in reducing inflammation. Therefore, researchers emphasize the study of dental nerve activity, especially in acute clinical problems in inflamed teeth and pulp regeneration. This review aims to investigate the possibility of using dental stem cells to regenerate dental nerves in order to repair dentin-pulp complexes for maintaining and restoring tooth structure and function, which nanosystems can help in this matter.
Materials and Methods:
In this paper, we review the literature regarding the theory of dental tissue engineering by neural differentiation of dental stem cells and nano-systems, and the comprehensive search on PubMed, Scopus, and Web of Science was conducted up to July 2022.
Results:
According to recent studies, dental soft and hard tissue healing also includes nerve fibers. A deeper understanding of how dental nerves are implicated in pulpitis may assist endodontic treatment. Stem cell-based treatments may be used to regenerate dental nerves to repair dentin-pulp complexes to maintain and restore tooth structure and function.
Conclusion:
The emphasis on dental nerve regeneration appears to be a critical stage in fostering spontaneous tooth regeneration as well as a sustainable tooth regeneration method. It is essential to further investigate dental tissue engineering by neural differentiation of dental stem cells.
1. INTRODUCTION
One of the most serious public health concerns is tooth decay and pulp inflammation, often known as pulpitis. Pulpitis may be diagnosed clinically as a toothache caused by an accumulation of polymorpholeukocytes in the pulp area [1, 2]. Toothache, which varies in intensity depending on the stimulus which stimulates the damaged tooth, is sensed by a cascade of sensory nerve impulses to the brain [3]. One of the functions of dental nerves is to provide sensory signals to the central nervous system (CNS), which causes a variety of responses in the body. Primary sensory fibers detect stimuli in the pulp of the tooth and initiate signal transmission [4]. According to sensory signals, dental nerve fibers are thought to be only responsible for delivering pain impulses in pulpitis [5-7]. However, recent studies have shown that they have also been linked to the regulation of inflammatory reactions.
It was proven that after tooth inflammation, micro- circulation and sensory nerve activation diminish inflammation [8-11]. Microcirculation is affected by pulp nerve activity. Therefore, researchers emphasize upon the study of dental nerve activity, especially in acute clinical problems in inflamed teeth and pulp regeneration [12, 13]. Also, nerve fibers are an important component of the dental pulp, especially during inflammation, which directs the tooth's essential functions, such as angiogenesis, rousing immune cells, preserving pulp structure, and strengthening defense mechanisms. In addition, neuropeptides derived from nerve regeneration may aid in repairing injured teeth [14, 15]. In other words, the regenerated dental nerve stimulates the soft tooth component (pulp) and aids in the formation of the hard tooth component [16].
Stem cell-based treatments may be used to regenerate dental nerves to repair dentin-pulp complexes to maintain and restore tooth structure and function [17, 18]. Dental stem cells (DSCs) are found in the dental pulp and help to regenerate and repair damaged teeth by reducing inflammation caused by caries. To rebuild dental pulp, all neural crest-derived DSCs are pluripotent and grow into endothelium, adipocyte, odontoblast, chondrocyte, myocyte, neural, and osteoblast cells [19-22]. In addition, all DSCs were shown to be able to differentiate into neuron-like cells in tissue engineering of various target organs, including the brain and spinal cord, as defined in vitro situations [23-26].
Apexification and dental pulp stem cells (DPSCs) implantation were explored by Xuan and colleagues, to treat pulp necrosis in immature teeth. After 12 months, DPSCs implantation restored three-dimensional pulp tissues with blood channels and sensory neurons. In comparison to apexification, DPSCs implantation enhanced root length and reduced apical foramen width. DPSCs also regenerate sensory nerves in the tooth pulp. According to the results, DPSCs implantation may be able to restore entire dental pulp and repair trauma-related tooth damage [27].
Tooth regenerative therapy via DSCs mainly depends on tissue engineering by utilizing nano-structured biomaterials [28, 29]. Using nano-designed materials, it can now concentrate numerous diverse activities in a small volume and improve targeting quality while lowering the cost and delivery of active molecules. In addition, multi-active therapies and nanomaterials with extracellular mimetic nanostructures are critical for resolving infection and inflammation while guiding pulp cell colonization and differentiation [30, 31].
It has been observed that human neural tissue's extracellular matrix has a nano-to-micro hierarchical structure. Today, nanotechnology is used in all medical fields [32-36]. Nano-systems are the only option to reconstruct human neural tissue extracellular matrices with outstanding biomimetic properties and physicochemical properties, which makes significant advances in neural tissue engineering [37]. Nano-system is produced from a variety of natural and synthetic biomaterials [38-40], such as collagen [41, 42], chitosan [43], gelatin [44], poly-ε-caprolactone (PCL) [45], poly (lactic-co-glycolic acid) (PLGA) [46], etc. that promote neural tissue formation. Unfortunately, the number of studies related to tooth reconstruction, pertaining neural regeneration is very limited. Most studies focused on DSCs regenerating the central/peripheral nervous system [47-50].
This review first discussed dental tissue engineering by neural differentiation of dental stem cells and the role of nano-system in the regeneration of nerve tissue in the system of teeth.
2. SEARCH STRATEGY
To include related articles and studies of neurogenerative in relevance to teeth restoration, narrative research was conducted on PubMed, Scopus, and Web of Science up to July 2022. The research only involved full-text articles published in English. For instance, the research syntax used on PubMed included “dental stem cells,” “dental Stem cells,” DSC or DSCs, Neurogenesis, “Neurogenesis,” or “tooth's nerves,” “tooth nerve” or “dental nerve,” and “tooth regeneration,” or “dental regeneration”, “tooth restoration,” “dental restoration,” and, “nano-systems,” or “nano-system,” “nanosystem”, “nanosystems,” “nanocomposite”, “nanocomposites,” and, “nerve differentiation,” and, “nanotechnology,” or “nanotech.”
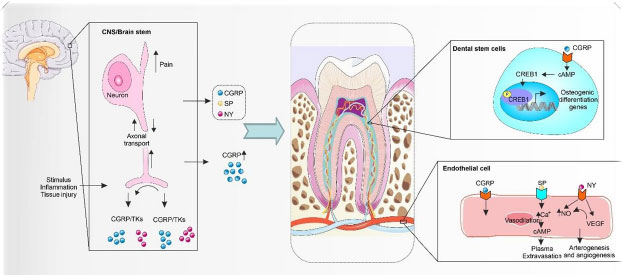
3. ROLE OF DENTAL NERVE IN TOOTH DEVELOPMENT
The dental pulp is a neural network composed of the dental nerve of the fifth branch of the cranial nerve, which includes both sensory and sympathetic nerves. The sensory nerves of the pulp are situated in the trigeminal ganglion, where thousands of axons enter the tooth through the apical foramen and branch in the whole pulp. Most neural packets enter the dentin and form the parietal network of nerves (plexus of Raschkow). The end of the free nerve is on the odontoblast cell layer. The nerves continue to the coronal region, create a plexus in the vicinity of the odontoblasts and eventually join the dentinal tubules. They have diverse functions, positions, and associations with the pulp, dentin, vasculature, and immune cells. The dental pulp nerve secretes some neuropeptides such as neuropeptide Y (NY), substance P (SP), calcitonin gene-related peptide (CGRP), vasoactive intestinal polypeptide (VIP), galanin, and pituitary adenylate cyclase-activating polypeptide (PACAP), neurokinin A (NKA), etc. which have a vital role in regulating some function in teeth such as dentinogenesis, vascularization, immune reaction, and bone regeneration [51-54] (Fig. 1).
3.1. Dentinogenesis (the formation of dentin)
Nerve fibers are involved in about 30 to 70% of the odontoblastic process, called intra-tubular nerves. Dentinal tubules include multiple nerve endings in the pre-dentin and dentin located at a distance of 100-150 micrometers from the pulp, which accompanies the odontoblast process [55]. CGRP is secreted from dental pulp nerves and is effective in dentin calcification [56]. According to another research, various concentrations of CGRP were studied on bone colonies. The results suggest that the higher concentration of this neuropeptide raises the ossification level [57]. The molecular mechanism of this reaction, CGRP, binds to target cell surface receptors that cause the expression of ossification-related genes [58]. Furthermore, the research found that CGRP plays a vital role in the creation of the dentin bridge during pulpotomy healing [59]. A post-pulpotomy study of first maxillary molars in 56-day-old Wistar rats showed that after seven days, large numbers of newly germinated CGRP-IR nerve fibers appeared in the remaining pulp, and some terminated in differentiating odontoblast layer and initial matrix layer of the dentin bridge were completed. These findings suggest that sensory neuropeptides such as CGRP may be involved in the formation of dentin bridges in the rat molar [59].
3.2. Regulation of Vascularization
Preservation of pulp function is due to microcirculation, which provides tissues with nutrients and removes metabolites and wastes. During this circulation, the blood pressure in the pulp vascular system is coordinated with the interstitial tissue, and sufficient blood pressure is maintained in the pulp vascular system. The intensity of blood flow in the pulp vessels varies throughout life, including growth, puberty, and especially inflammation. In the first stage of inflammation, the intensity of blood flow and vascular permeability increases. Then, leukocytes migrate to the venular network [60, 61]. Under these conditions, the human pulp can see sufficient oxygen and nutrients for damaged tissue. Observations have shown that pulp blood vessels are functionally related to nerve fibers [62]. Studies have shown that the rate of blood flow in the anterior or molar teeth of rats exposed to electrical stimulation increases, whereas if the inferior alveolar nerve is denervated, the rate of blood flow to the stimulus decreases [63-65]. NY is released by the dental pulp nerve, which is situated near blood vessels and reduces blood flow from the pulp via a calcium-dependent process.
In contrast, increased expression of NY regulates angiogenesis factors and repairs pulpal inflammation. Besides, interdental sensory nerves provide neuropeptides, such as CGRP and SP, that facilitate blood flow from the pulp [66-68]. In addition to neuropeptides secreted by sensory nerves, glutamate can regulate blood flow in vasodilation. Glutamate transporter-expressing neurons release glutamate into the pulp [69], which binds to the pulp's metabotropic glutamate receptors (GluRs), which regulate pulp blood flow [70].
As a result, neuropeptides secreted from the dental nerve have a critical role in regulating tooth function. Loss of nerve in the tooth stops blood flow to the tooth pulp and causes the dentin to change color (yellow, brown, even black), sometimes referred to as pulp necrosis, leading to tooth death. Different trauma studies in animals and humans have revealed that nerve fibers respond to dentin and pulp damage by germinating their terminal branches in the adjacent residual pulp and altering their neuropeptides. Therefore, a few days after injury, the undamaged nerve fibers return close to their original shape and function. Its feature has the role of restoring dental function [71-74]. El Karim et al. discovered that culturing human pulp fibroblasts in the presence of neuropeptides such as calcitonin gene-related peptides increased the expression of angiogenin and VEGF proteins. This is the first study to show that dental neuropeptides play a role in regulating angiogenic growth factor expression, implying that neuropeptides may play an important role in pulpal inflammation and repair in the future [66].
3.3. Regulation of Immune Reaction
Dendritic cells (DCs) expressing class II antigens in the periodontoblastic region of the dental pulp (OX6 +) are closely related to the nerve section of the tooth [75]. When tooth decay progresses to the dentin area and is not treated, DC cells and nerve cells in the tooth decay area move precisely at the pulp-dentin border [76]. At the level of myelinated nerve fibers in the dental pulp, there is a toll-like receptor (TLR)-4 and cluster of differentiation 14 (CD14), which are known as the first line of defense against infection and critical molecules in the innate immune system, respectively [77, 78]. The spatial similarity of the nerve endings to the DC cells indicates an almost similar function, suggesting the possibility of paracrine nerve interaction and immunity in the early stages of carious pulpitis. Therefore, the dental nerve is crucial for the dental immune system. A recent bioinformatics study was conducted to identify possible pulpitis biomarkers. Immune system signaling pathways such as the IL-7 signaling pathway, the Toll-like receptor signaling pathway, the NF-kappa B signaling pathway, and the TNF signaling pathway are activated during dental pulp inflammation, as are neuroactive ligand-receptor interactions [79].
Observations show that the activation of trigeminal sensory neurons combined with the sensitivity of transient receptor potential vanilloid subtype 1 (TRPV1) is mediated by the activation of the TLR4-mediated mechanism by lipopolysaccharides (LPS) microorganisms [80, 81]. This response lowers the activation threshold of nerve cells and leads to a hyperalgesic state. As previously described, neuropeptides are involved in regulating localized microcirculation in pulpitis. It promotes and directs immune cells to local tissues by increasing blood flow and vascular permeability. It has been observed that the denervated molar pulps of mice weaken the immune system in the inflammatory tissue of the pulp compared to healthy teeth [82, 83]. Ho et al. showed that at the surface of immune cells, such as monocytes/macrophages, there are receptors for receiving neuronal signals [84]; as a result, the tooth nerves can recruit immune cells directly. A recent study showed that macrophages, as inflammatory and immunologic mediators in the trigeminal ganglion, play a major part in the development of pulpitis and hyperalgesia according to the processes of CNS underpinning ectopic pain after peripheral inflammation [85]. Various studies have shown that the neuropeptides SP and CGRP interact with human monocytes and T lymphocytes through chemical mechanisms [86, 87].
Following dental pulp injury, neuropeptides enhance vascular permeability and promote immune cell chemotaxis into the tooth pulp by direct contact with immune cells. If the dental nerve is destroyed, there is no longer a stimulus to invoke immune cells [82, 83]. Although, in healthy teeth, neuropeptide production and secretion decrease (35), it can be concluded that the main factor in maintaining the number of immune cells in healthy tooth pulp is not nerve cells.
3.4. The Role of Nerves in Apical Periodontitis
Inflammation of the teeth and pulpitis can affect periapical tissue and eventually lead to acute/chronic apical periodontitis. Reports have shown that the sensory nerves of the tooth gather in the periapical lesions and recruit macrophages and dendritic cells in periapical tissue. Thus, Dental nerves are essential in initiating the immune response and tissue repair in apical periodontitis [88, 89]. Apical periodontitis is associated with bone destruction in the periapical region, in which the sprouting of dental nerves for bone regeneration is observed [72, 90].
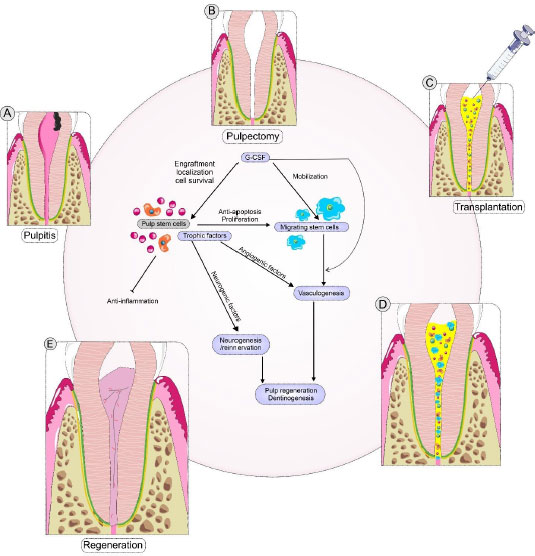
3.5. Reconstruction of the Dental Nerve in the Tooth Regeneration
Pulp/dentin reconstruction is one of the most critical challenges and the ultimate goal for functional tooth restoration. Tooth innervation can maintain dental pulp homeostasis. Tooth sensory nerves can stimulate the pulp's defense system by invoking immune and inflammatory cells to the injury site [65]. In the sensory denervation of damaged teeth, blood flow and immune cell recruitment are disrupted, resulting in necrosis of the tooth pulp [91, 92]. Fristad et al., by intermittent stimulation of the rat pulp, observed that re-innervation/neurogenesis improves coronal dentin [65]. Ihara et al. examined pulp stem cells with granulocyte stimulating factor (G-CSF) in dog teeth to regenerate tooth pulp. The results showed that the nerves labeled DiI to the trigeminal ganglion responded positively to the electrical pulp test, and the sensory signals were felt as pain. Neurogenesis axons can aid in the process of angiogenesis, the release of immune cells, and the regulation of inflammation in damaged tissue to strengthen the pulp's defense mechanism (Fig. 2) [93]. Also, the blood flow in the regenerated pulp after two months was similar to that of a normal pulp, which could play an essential role in regenerating the pulp and dentin by controlling inflammation. The interaction of nerve fibers and blood flow maintains pulp tissue homeostasis, which can cause the enlarged apical portion to fade after pulpectomy and the formation of lateral dentin, which is a benefit in inhibiting tooth fractures. This restoration prevents secondary tooth decay and prolongs tooth life [93].
3.6. DSCs and Differentiated Neuronal Markers
Depending on their origin, DSCs inherit some of the typical features of nerve cells [94, 95]. DSCs inherit some of the typical features of nerve cells, depending on their origin. DSCs have been displayed to be able to express specific neuronal markers, for example, glial fibrillary acidic protein (GFAP), S100 calcium-binding protein B (S100B), and nestin. In addition, DSCs express more neuronal markers than bone marrow mesenchymal stem cells (BMSCs), indicating that DSCs can be utilized as perfect cells for neural differentiation and regeneration. Along with neuronal markers, the expression of neurotrophic factors is essential. Neurotrophic factors such as glial cell line-derived neurotrophic factor (GDNF), brain-derived neurotrophic factor (BDNF), and nerve growth factor (NGF) can be expressed through various DSCs. The prepared conditioning media from DSCs can induce neurite outgrowth and improve the growth rate of Schwann cells in vitro [96]. In addition, DSCs, unlike other somatic stem cells, display expression of embryonic stem cells (ESC) pluripotency markers, such as stage-specific embryonic antigen-4 (SSEA-4), octamer-binding transcription factor 4 (Oct-4), tumor rejection antigen (TRA)-1–60 and, Nanog, when derived from the immature dental pulp during the development of the tooth.
DSCs have neuron-like characteristics, which makes in vitro differentiation easier. Several methods for DSC-to-neuron differentiation have been developed. In summary, inductive protocols need to DSCs versatile differentiation. DSCs can be differentiated into neurons, Schwann cells, oligodendrocytes, and dopaminergic-like cells. Therefore, DSCs are suitable stem cell sources to treat nervous diseases in stem cell therapy. The efficiency of nervous disease therapies with stem cells is strongly influenced by trophic factors such as GDNF, BDNF, NT-3, NGF, platelet-derived growth factor (PDGF), and vascular endothelial growth factor (VEGF) [97, 98]. The DSCs express these trophic factors more than MSCs derived from adipose tissue (ADSCs) and bone marrow (BMSCs) [97, 99]. Furthermore, DSCs display more neural supportive and neuroprotective properties than other MSCs in response to nervous system damage. DSCs possess the capability to decrease neurodegeneration by the secretion of NGF and BDNF in the neuronal apoptosis in early stages and stimulate the survival of motor and sensory neurons in spinal cord injury (SCI) [100, 101].
Additionally, secreted trophic factors via DSCs promoted the regeneration of axons despite the attendance of inhibitors of axon growth in the entirely transected spinal cord model of SCI [102, 103]. DSCs also secreted cytoprotective factors, providing direct and indirect protection against cell death in an ischemic astrocyte model of injury [104, 105]. DPSCs have displayed a higher cytokine expression facilitating neural differentiation compared with other stem cells, such as stem cells from the apical papilla (SCAP), Dental follicle stem cells (DFSCs), and BMSCs [106]. SCAP showed neural differentiation activity. During tooth development, the dental papilla converts to dental pulp tissue. Findings suggest that SCAP characteristics in terms of maturity are more similar to ectomesenchyme than DPSCs [107].
SCAP was derived from the apical papilla tissue of immature permanent human teeth, whose proliferation activity is two or three times more than DPSCs. These SCAPs express several neuronal markers such as βIII-tubulin, nestin, GFAP, and neurofilament M upon stimulation of neural differentiation with EGF and bFGF [108]. DFSCs are isolated from wisdom teeth and have this multilineage differentiation capability [109]. Morszeck et al. reported that DFSCs could form neurosphere-like structures in serum-free media comprising FGF-2 and EGF plated onto low-attachment cell culture plates. Their study also showed that DFSCs-derived neural cells grown on gelatin and laminin show a relatively long axon-like cell extension, showing that the morphology of neural cells and axon-like cell extensions were related to cell culture substrates. In addition, they showed that the time-dependent addition of supplements to neural stem cells, such as retinoic acid, FGF-2, and EGF, affected the neuronal cell lineage differentiation into γ-aminobutyric acid -ergic (GABAergic) or dopaminergic neurons [109].
Inflammatory cytokines may both aid and impede the management of neurogenesis. IL-6 is a neuromodulator that affects brain stem cell renewal, progenitor cell division, and differentiation [110]. 13 IL-4, IL-11, and IFN-g all promote increased neuronal differentiation [111]. SHED displayed a predominance expression of IL-6, IFN-g, and IL-4 in this study, but DPSCs revealed a 1.34-fold greater expression of IL-11 than SHED [112].
SHED showed substantially greater amounts of cytokines related to neuroprotective factor synthesis than DPSCs. Among these cytokines are NT-3, BDNF, and GDNF. There is evidence that genetically altered cells may live longer and operate better, and that overexpressing neuroprotective proteins or neurotransmitters in cells might allow more rapid neural regeneration [113-115].
Among the neurogenesis-related cytokines, b-NGF expression was 6.85 times higher in DPSCs than in SHED [112]. NGF, a member of the protein family known as neurotrophins, is an important regulator of neurodevelopment and cell proliferation. In previous studies, it was found to be able to repair nerve injury in clinical therapy; Phyo et al. reported that increased levels of NGF expression supported reinnervation in pulp regeneration in a rat molar [116]. When employed as paracrine factors, NT-3, GDNF, and BDNF, which were shown to be more abundant in SHED than in DPSCs, were found to stimulate neuronal differentiation in both SHED and DPSCs. This enabled SHED to provide a more immunologically tolerant environment for stem cell transplantation, as well as superior circumstances for cell proliferation and neurogenesis. DPSCs, on the other hand, have been promoted as beneficial for angiogenesis.
The PDLSC secretome may increase the functioning of the PI3K/Akt/mTOR axis, leading to the restoration of BDNF production and the reduction of oxidative stress and inflammation in injured neurons. Furthermore, the presence of neuroprotective chemicals such as NT-3, IL-10, growth factors, and immunomodulatory cytokines in the conditioned medium provides a neuroprotective effect [117]. Furthermore, prior research indicated that the SCAP had much greater amounts of BDNF and GDNF than the other DSCs [103].
3.7. Nano-system Involved in Dental Nerve Differentiation and other Neural Tissue
One of the promising new therapies in the field of endodontics is dentin–pulp complex tissue engineering. The materials employed in the field of regenerative endodontics now, for instance, mineral trioxide and calcium hydroxide aggregates, cannot guarantee the entire dentin–pulp complex regeneration, particularly about neurovascular induction. A water-soluble graphene oxide–copper (GO-Cu) nanocomposite was fabricated by Li et al. [118], who studied its neurovascularization and odontogenic-inducing potentials via DSCs. Low doses (≤10 µg/mL) of graphene oxide–copper was ineffective on DPSCs’ promoted adhesion, proliferation, and viability. The treatment of DSCs with graphene oxide–copper led them to differentiate into odontoblasts and secret higher quantities of GDNF and VEGF at the time when cultured on a scaffold made of calcium phosphate cement (CPC/GO-Cu) coated with graphene oxide–copper. The treatment of human umbilical vein endothelial cells (HUVECS) with graphene oxide–copper led to more substantial migratory potential, tube formation, and higher vascular endothelial growth factor expression. Additionally, using the graphene oxide–copper treatment, the immunomodulatory genes, such as hepatocyte growth factor (HGF), 2,3-dioxygenase (IDO), and human leukocyte antigen G (HLA-G), were upregulated in DPSCs. The subcutaneous transplantation of calcium phosphate cement/graphene oxide–copper into nude mice led to the formation of dentin–pulp complex–like structures expressing CD31, growth-associated protein 43 (GAP43), and dentin sialophosphoprotein (DSPP), and the blood vessel numbers and the mineralized area were lower in the CPC-alone group than the CPC/GO-Cu group. These results reflect graphene oxide- copper's neurovascularization and odontogenic-inducing potentials and its promising applicability for regenerative endodontics purposes [118].
They reflected the graphene oxide–copper biocompatibility and its neurovascularization-inducing and odontogenic behaviors toward DPSCs. As a Cell Counting Kit-8 (CCK-8) assay, EdU incorporation and live/dead staining have affirmed that at low doses of GO-Cu, DSCs proliferate and survive actively. As the formation of the calcified nodule and odontogenic genes’ protein and mRNA expression have shown, grapheme oxide–copper may lead to promoted odontogenic differentiation of DPSCs. Improved migration, greater VEGF expression of HUVECs, higher tubular-structure formation, and higher expression of GDNF and VEGF in DSCs confirm the neurovascularization-inducing potential. In addition, graphene oxide–copper leads to increased expression of immunomodulatory genes HGF, IDO, and HLA-G in DPSCs. Eight weeks after the subcutaneous transplantation of CPC/ GO-Cu loaded with DPSCs, dentin–pulp complex–-structures are recreated in the defect region, and immunofluorescence staining reflects CD31, GAP43, and DSPP expression within the newly regenerated tissues. This research showed graphene oxide- copper's neurovascularization and odontogenic-inducing effects on DSCs, a new promising dental material used in regenerative endodontics [118].
The aligned electrospun PCL/PLGA material could be employed as a scaffold for spinal cord restoration. The inserted DFCSs onto PCL/PLGA can cause differentiation into neural cells in lesions of SCI. There is a hypothesis that aligned electrospun fibers have the potential to aid the spinal cord structure as well as cause the differentiation of neural cells [119]. A brand-new biomaterial called chitosan scaffolds has proved to facilitate inducing DSCs to turn into neural cells and can possibly be utilized in SCI therapy in the future [120]. Heparin-poloxamer (HP), can simply create a gel at body temperature as a thermosensitive hydrogel; therefore, it is appropriate for in vivo treatments. It is revealed that nerve lesions could be repaired when HP is loaded with growth factors, namely FGF and NGF. This is achieved through activation of the Janus kinase (JAK)-signal transducer and activator of transcription (STAT), the mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase 1/2 (ERK), The phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) and signaling pathways [121]. The combination of bFGF and DPSCs remarkably affected neuronal reproduction, recovery of function, and spinal cord tissue reconstruction [122]. According to statistical analyses, HP-bFGF-DPSCs outperform HP alone or HP-bFGF in nerve reconstruction [122]. DSC-based treatment will have a promising future for the treatment of SCI. Various studies showed that collagen scaffolds combined with neural-induced DPSCs could foster axonal outgrowth and myelination. The DPSCs could also be induced to differentiate into functional oligodendrocytes by increasing the exogenous gene expression of the oligodendrocyte lineage transcription factor 2 (OLIG2). This presented a therapeutic capability in sciatic nerve injury [123].
4. LIMITATIONS IN DENTAL TISSUE ENGINEERING AFTER IMPLANTING
Painful post-traumatic trigeminal neuropathy (PTTN) is a known complication of dental implant therapy. PTTN is a painful condition that may result from injury to the sensory division of the trigeminal nerve [124, 125]. Treatment of this condition is challenging and there is presently no established standard of treatment for managing neuropathic pain. Another challenge in tissue engineering is rejection. When the immune system recognizes the transplant as foreign, it rejects it, triggering a chain reaction that finally causes the transplanted organ or tissue to be destroyed. Cytokines, soluble molecules generated by many types of immunocompetent cells that impact each other, play an important role in the immune response [126]. The immunological responses were studied from two perspectives: cellular and humoral.
4.1. Cellular Immunity
When the body is exposed to implant material, macrophages are the first phagocytes to get activated, resulting in the early stages of inflammation [127, 128]. The presence of macrophages in rejected implants was connected to the formation of granulation tissue, suggesting a connection between the two processes. Titanium metal particles were found in the cytoplasm of macrophages around the site of granulation tissue [129]. Moreover, several studies have shown that the presence of M2 macrophages in peri-implant tissue is associated with decreased inflammation, improved wound healing, and effective implant osseointegration [130, 131].
Dendritic cells are involved in the cellular immune response. However, more study is needed to discover the particular influence of DCs on dental implant rejection in order to achieve a positive outcome. Langerhans cells (LC), a kind of DC, were found in the stratified epithelium, such as the skin's epidermis and the mucosa lining the mouth. They have the ability to modify the immune milieu of the oral mucosa as well as preserve oral tissues during infection [132]. Gooty et al. also found that the number of LCs (factor CD1a) in the epithelium and lamina propria of healthy mucosa collected prior to implant placement was considerably greater than healthy peri-implant mucosa acquired at the time of gingival former implantation. The researchers concluded that diminished immune responses were caused by a smaller amount of LCs CD1a in the peri-implant tissue [133]. According to research, the quantity of mature LCs is decreasing as a result of dental implant installation. The implants, on the other hand, have a negative impact on precursor LCs and may increase their quantity in peri-implant tissue.
4.2. Humoral Immunity
Cytokines, growth factors, and hormones are only a handful of the molecules that govern immune system cell communication [134]. Elevated IL-1 levels have been associated with dental implant failure, presenting a full picture of host reactivity in patients with peri-implantitis collapse [135]. Furthermore, IL-1 is acknowledged as an important factor in differentiating between healthy implants and peri-implantitis [136, 137]. TNF- activates the significant mediators of inflammation when coupled with IL-1 [138]. Chronic inflammation, such as peri-implantitis and periodontitis, raises tumor necrosis factor-alpha levels. Extreme TNF- production, as shown by our findings [139], is a significant clinical issue for acute and chronic inflammatory diseases. Patients with failed implants exhibited considerably greater TNF- levels than those with successful implants. This TNF increase has been linked to the failure of dental implants [126, 136, 140-145].
Researchers discovered that titanium dental implants boost the synthesis and release of proinflammatory cytokines such as interleukin (IL)-1, tumor necrosis factor-alpha, interleukin (IL)-17, interleukin (IL)-6, interleukin (IL)-8, and interleukin (IL)-2. These upregulations may be linked to implant failure and osseointegration loss. However, various studies have shown contradictory results about the rise in these cytokines. More study is needed to identify the real role of these substances in dental implant rejection [146].
Interleukin-10 (IL-10) is a versatile cytokine that may affect a variety of hematopoietic cells. This cytokine's principal role is to inhibit or stop the inflammatory response. Because of its favorable in vivo regulatory influence, capacity to reduce acute inflammatory feedback, and ability to produce a negative feedback loop that lowers the release of inflammatory mediators [147], IL-10 is a crucial immune modulator [148]. Inhibiting the production of inflammatory cytokines such as IL-6 and TNF- may reduce macrophage and Th17 response activity [149]. TGF-ß1 may be involved in the control of inflammation and immune suppression [150]. Important in wound healing and the local inflammatory response [127].
CONCLUSION
In this review, the complex role of the dental nerve in regenerating dentin and regulating the uptake of immune cells in pulp inflammation and tooth decay was discussed, which goes beyond the traditional view of nerve function only to transmit pain signals. These advances can provide abundant information about nerve and pulp function. Therefore, accurate knowledge of dental pulp neurology and regulatory mechanisms of pulp nerve network can be new therapeutic perspectives in endodontics and the development of approaches to target dental pulp neurons.
Nanotechnology for tissue engineering applications has made significant advances in the regeneration of bone, cartilage, arteries, nerves, and bladder tissue. However, the number of nanotechnology studies in pulp nerve regeneration has been minimal. Furthermore, the use of the unique properties of synthetic nanomaterials in extracellular matrix simulation (mimicking in vivo situations) has been neglected. Therefore, we propose that future studies focus on the cellular and molecular mechanisms of the nervous system in the context of nano-systems like nanocomposite to identify unknown parts of the role of the pulp nerve in tooth regeneration.
LIST OF ABBREVIATIONS
| CNS | = Central nervous system |
| DSCs | = Dental stem cells |
| DPSCs | = Dental pulp stem cells |
| NY | = Neuropeptide Y |
| TRPV1 | = Transient receptor potential vanilloid subtype 1 |
FUNDING
This paper was funded with support from the Vice-Chancellor for Research at Tabriz University of Medical Sciences under grant number 72936.
CONFLICT OF INTEREST
Drs. Simin Sharifi and Solmaz Maleki Dizaj are on the Editorial Advisory Board of The Open Dentistry Journal.
ACKNOWLEDGEMENTS
Declared none.


