The Effect of 10% Sodium Ascorbate and Er: YAG Laser on the Microtensile Bond Strength of Composite Resin to Bleached Enamel
Abstract
Background:
There are various methods proposed to prevent the reduction of substrate micro tensile bond strength to bleached enamel. Therefore, this study aims to evaluate and compare the effectiveness of two common methods, namely 10% sodium ascorbate and Er:YAG laser irradiation, in increasing the microtensile bond strength of composite resins to bleached enamel.
Methods:
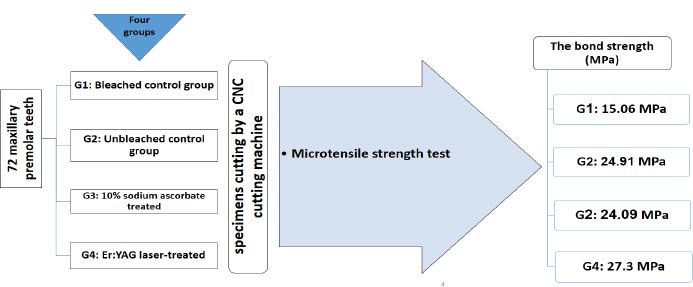
In this in vitro study, 72 maxillary premolar teeth were divided into four equal groups: bleached control group, unbleached control group, treated with 10% sodium ascorbate, and Er:YAG laser-treated group (60 mJ, frequency = 10 Hz, and power of 0.6 W with a 400-µm diameter tip). The samples were cut by a CNC cutting machine for a microtensile bond strength test.
Results:
The Er:YAG laser group showed the highest mean bond strength (27.3 MPa), while the bleached control group had the lowest (15.06 Mpa). There was a significant difference between the bleached and unbleached control groups (P<0.001). Samples prepared with 10% sodium ascorbate and the Er:YAG group had higher tensile bond strength than the bleached control group (P<0.0001). Tensile bond strengths were not significantly different between the 10% sodium ascorbate and the Er:YAG laser preparation groups (P=0.361).
Conclusion:
The findings of this study indicate that the microtensile bond strength of composite resins to bleached enamel can be increased by 10% sodium ascorbate and Er:YAG laser irradiation.
1. INTRODUCTION
The increasing demand for dental health, aesthetics, and a beautiful smile has led to advancements in tooth-bleaching procedures [ 1]. Two techniques for bleaching vital teeth are available: in-office bleaching under the supervision of a dentist and at-home bleaching using a nightguard. Carbamide peroxide is commonly used in the at-home technique, while hydrogen peroxide is the most effective agent for eliminating intrinsic stains in the office [ 2]. However, both techniques induce morphological changes in the bleached enamel, increasing tissue porosity and permeability and affecting bond strength due to problems with adhesive penetration [ 3, 4]. Various techniques have been proposed to prevent decreases in substrate bond strength to bleached enamel, including delayed bonding, abrading the surface enamel, using acetone-containing adhesives, applying alcohol to the tooth surface, and using sodium ascorbate gel or solution as an antioxidative agent. Waiting for one day or three weeks is the most common and easiest method [ 5-7]. However, since patients come from different regions or have time limitations, it is necessary to carry out adhesive restorative procedures in the same session [ 8-10]. To shorten the waiting time, a 10% sodium ascorbate gel is applied to the bleached enamel surface to accelerate the removal of the residual oxygen layer and restore enamel adhesion to pre-bleaching values [ 10, 11]. It has been reported that applying 10% sodium ascorbate gel for 60 minutes can restore enamel bond strength in vitro, in situ, and in the clinic [ 12-14].
The advent of new technologies, such as lasers, has resulted in significant advantages in modern dentistry. Lasers can be used in restorative dentistry for cavity preparation, treating dentin sensitivity, tooth bleaching, composite resin polymerization, preparation of organic and inorganic tooth structures, and induction of surface changes in biomaterials [ 14, 15]. Erbium lasers are widely used by dentists. Er:YAG laser has a wavelength of 490 nm, has proper absorption by hydroxyapatite and water, and is very suitable for application to dental structures [ 14, 15]. The reducing effects of Er:YAG laser on tooth structures affect the bonding process after tooth bleaching procedures due to an increase in the temperature of the substrate and morphologic changes in the dentin and enamel structure. In addition, laser therapy makes it possible to immediately place the restorative materials by removing the residual free radicals and immediately neutralizing their effects [ 15].
Sodium ascorbate as an antioxidant and laser treatment with its ability to eliminate free radicals effectively increase the bond strength of adhesive agents to bleached enamel. However, few studies have investigated the effectiveness of these two techniques in improving the bond strength of composite resin to enamel after in-office bleaching. Therefore, the present study compared the effect of 10% sodium ascorbate and Er:YAG laser on the microtensile strength of composite resin on enamel bleached with 35% hydrogen peroxide [ 16].
2. MATERIALS AND METHODS
2.1. Study Population
The present study was conducted on 72 maxillary premolar teeth that were free from cracks, caries, or coronal restorations. The study was approved by the ethical committee of Ahvaz University of Medical Sciences (IR.AJUMS.REC.1397.243). Based on previous studies [ 17-20], a sample size of 18 patients was included in each group, with a 95% confidence interval and a study power of 90%. Therefore, a total of 72 teeth were included in the present study.
2.2. Study Protocol
The teeth were cleaned using periodontal curettes (Juya Instruments, PVT) and disinfected in 10% formalin solution (Kimia Daru, Iran) for one week at room temperature. They were kept in sterilized water for another week before the study began. A slurry of pumice (Pumice Medium, Sina Bartar) and a rubber cup (Brasseler, USA) were used to remove all deposits and impurities from the tooth surfaces. The roots were separated from the crown at the cemento-enamel junction (CEJ) using a diamond bur (Tizkavan, Iran) in a high-speed dental handpiece (NSK, USA) under air and water spray. The coronal pulp was removed, and the pulp chamber was filled with light-body Speedex impression material (Coltene, Switzerland) to prevent acrylic resin monomer (Meliodent, Heraeuse, Kuzeler, Germany) from entering the chamber.
The specimens were placed labially within translucent acrylic resin molds measuring 25×53×10 mm and immersed in cold water for curing. A bleaching gel (Opalescence, UT, USA) containing 40% hydrogen peroxide gel was applied to the enamel surfaces of the specimens for 20 minutes. The specimens were then rinsed and dried with water or air spray.
The specimens in the first group were treated with a 10% sodium ascorbate gel (ICN Biomedicals, USA). The gel was applied to the specimens for 15 minutes prior to the restorative procedures [ 21]. The standard amount of gel used in a tray, measuring 3 mm in thickness, was applied to the enamel surface. Specimens in the second group were treated with an Er:YAG laser (Kavo Biberach, Germany) using 60 mJ energy, a frequency of 10 Hz, and a power of 0.6 W with a tip diameter of 400 µm. The tip was placed perpendicular to the surface at a distance of 1 mm and moved in a transverse circling motion from the center of the tooth sample toward the peripheral areas for 5 seconds. A spray consisting of 60% air and 50% water under a pressure of 2 bars was used during the procedure.
The bleached control group did not undergo surface preparation and was etched and bonded after applying the bleaching gel. Composite resin samples were then placed on them, similar to the other groups. The unbleached control group was only etched and bonded, and composite resin was placed on the samples (Fig 1).
To apply composite resin (light-cured, 3M ESPE, Z250, USA) to the specimens, a translucent cylindrical mold with a diameter of 4 mm and a height of 6 mm was affixed to the specimen surfaces. Next, 35% phosphoric acid gel (Voco, Cuxhaven, Germany) was applied to the surface of all specimens for 20 seconds, rinsed for 15 seconds, and dried for 10 seconds. Then, a fifth-generation bonding agent (Single Bond 2, USA) was applied in two coats with a microbrush to the etched surfaces of the specimens, dried for 5 seconds with air spray, and cured for 20 seconds from a distance of 1 mm using a light curing device (Woodpecker, China). Z250 composite resin (3M ESPE, USA) was applied to the molds using the incremental technique to a thickness of 1 mm and light cured from both sides of the mold. After removing the mold, the specimens were stored in distilled water at room temperature for 24 hours to complete the polymerization process.
Next, the specimens were cut using a CNC cutting machine to prepare for the microtensile strength test, which was performed using a microtensile strength tester (Microtensile Tester, Bisco, Schaumburg, IL, USA). The device was set to a force of 50 kg at a strain rate of 0.5 mm/min. The bond strength (MPa) was calculated by dividing the force at failure (N) by the bonded surface area (mm 2).
3. RESULTS
3.1. Characteristics of the Study Population
Table 1 presents the primary data, with the highest mean micro tensile bond strength values observed in the group prepared with Er:YAG laser (27.3 MPa) and the lowest values in the bleached control group (15.06 MPa). According to ANOVA test, the mean shear adhesion values differed significantly among the four study groups (P < 0.001). The mean shear bond strength value in the group bleached with 40% hydrogen peroxide gel was significantly lower than in the unbleached and bleached control groups (P < 0.001).
| Groups |
Mean (MPa) |
SD |
Max (MPa) |
Min (MPa) |
| Gp + (n=18) | 24.91 | 5.845 | 35.25 | 15.27 |
| Gp – (n=18) | 15.06 | 4.538 | 21.93 | 7.960 |
| Sodium Ascorbate 10% (n=18) | 24.09 | 5.186 | 30.97 | 9.8 |
| Er:YAG laser (n=18) | 27.30 | 4.061 | 33.89 | 17.63 |
GP –: bleached control.
SD: standard deviations.
Min: minimum, Max: maximum.
There was a significant difference between the bleached control group and the group prepared with 10% sodium ascorbate (P < 0.001), with the microtensile strength higher in the latter. Similarly, there was a significant difference between the bleached control group and the Er:YAG laser-prepared group (P < 0.001), with the laser group having higher microtensile bond strength.
However, there was no significant difference between the unbleached control group and the group prepared with 10% sodium ascorbate (P=0.905) or between the unbleached control group and the group prepared with Er:YAG laser (P=0.768). Nonetheless, preparation with Er:YAG laser significantly increased microtensile strength.
There was no significant difference between the group prepared with 10% sodium ascorbate and the Er:YAG laser-prepared group (P=0.361). However, the results indicate that preparation with Er:YAG laser significantly increased microtensile bond strength compared to preparation with 10% sodium ascorbate (Table 2).
| Group comparison | The mean MPa in group 1 | The mean MPa in group 2 | Mean difference | The mean MPa difference at 95% confidence interval | p. value |
| Gp + vs. Gp – | 24.91 | 15.06 | 9.847 | 5.499 to 14.20 | <0.001 |
| Gp – vs. 10% sodium ascorbate | 15.06 | 24.09 | -9.027 | -13.38 to -4.678 | <0.001 |
| Gp – vs. Er:YAG laser | 15.06 | 27.30 | -12.23 | -16.58 to -7.886 | <0.001 |
| Gp + vs. 10% sodium ascorbate | 24.91 | 24.09 | 0.8206 | -3.528 to 5.169 | 0.3 |
| Gp + vs. Er:YAG laser | 24.91 | 27.30 | -2.387 | -6.736 to 1.961 | 0.2 |
| 10% sodium ascorbate vs. Er:YAG laser | 24.09 | 27.30 | -3.208 | -7.556 to 1.141 | 0.3 |
GP –: bleached control.
NS: Not significant.
4. DISCUSSION
The current study is consistent with previous research indicating that tooth bleaching reduces microtensile bond strength. However, this effect can be mitigated by surface preparation using 10% sodium ascorbate or Er:YAG laser irradiation. Furthermore, the results demonstrate that there is no significant difference in micro tensile bond strength between bleached teeth and those treated with sodium ascorbate or Er:YAG laser. It should be noted that a limitation of this study is that the underlying mechanism of these materials was not assessed by examining any induced morphological changes.
The present study found that microtensile bond strength decreased significantly after bleaching with 40% hydrogen peroxide gel, and the microtensile bond strength in the bleached control group was significantly lower than that in the unbleached control group. Several studies have reported a decreased bond strength of composite resin to tooth structure after bleaching procedures. Rego et al. studied 20 sound human premolar teeth and reported that bleaching with 40% hydrogen peroxide significantly decreased the microtensile bond strength [ 22]. In addition, Iska et al. showed that bleaching decreased the microtensile bond strength [ 23]. A bleaching procedure involves an oxidizing agent, which can leave oxygen and hydrogen peroxide within the tooth structure, preventing complete penetration of resin and polymerization of resins cured by a free radical mechanism [ 24, 25]. Delaying the restorative procedure after bleaching is a simple method to resolve this issue. Therefore, it has been suggested that bleached teeth be restored after a delay of one week [ 26, 27]. However, different time intervals have been suggested, with some studies suggesting a 3-week delay [ 28, 29] and Van der Vyver suggesting a delay of at least two weeks [ 30]. Therefore, there is no consensus on the duration of the delay [ 30].
However, some patients may not be able to comply with this delay due to various reasons, and it is desirable to reduce the time required for treatment. To increase bond strength immediately after the bleaching procedure, several techniques have been suggested by different researchers. Barghi et al. reported that using solutions such as alcohol, acetone, or acetone-containing bonding agents instead of water can decrease the effect of bleaching agents on the bond strength of composite resin to enamel [ 8]. Khalili et al. found that alcohol decreased residual water and oxygen, thereby increasing the bond strength of composite resin to bleached enamel [ 31]. Sung et al. recommended the use of Opti Bond, an alcohol-based bonding agent, for bonding after tooth bleaching [ 31]. However, some studies have shown that the type of solvent does not affect the efficacy of the bonding agent after bleaching, and using ethanol-based or acetone-based bonding agents may decrease the bond strength of composite resin [ 8, 32].
Since free radicals derived from oxygen after tooth bleaching may injure periodontal tissues and initiate inflammatory responses and external root resorption, some researchers have suggested using antioxidative agents such as catalase scavenger enzymes or free radicals such as ascorbic acid, alpha-tocopherol, glutathione peroxidase, and salicylic acid derivatives to neutralize these free radicals. This prevents these toxic agents from damaging teeth [ 33]. Therefore, considering the ability of these materials to neutralize free radicals, it appears that using these agents can significantly increase the bond strength of composite resin to enamel after bleaching procedures, in addition to the effects mentioned above.
Ascorbic acid and its salts have antioxidative potential and are widely used in the food industry. It appears that they have no detrimental biological effects or clinical risks. These potent reducing agents can release two high-energy electrons, and this electron-emitting property reduces oxidized dentin and enamel. These agents have positively affected some dental treatments, such as endodontics. In a study by Mitzi et al., 10% ascorbic acid or 10% sodium ascorbate for 10 minutes after root canal irrigation with sodium hypochlorite solution during root canal treatment increased the bond strength to dentin. The study also showed that applying 10% sodium ascorbate immediately after bleaching increased microtensile bond strength significantly [ 34], with significant differences between the bleached and unbleached control groups and the group prepared with sodium ascorbate [ 34]. One study evaluated the effect of different antioxidant agents on the microtensile bond strengths of composite restorations to enamel bleached with 38% hydrogen peroxide. Finally, they found that Bleaching with 38% hydrogen peroxide reduced the bond strength of composite to enamel [ 35]. In a related study, Ferreira et al. examined the impact of catalase, 2% chlorhexidine gel, and 1% sodium hypochlorite on the microtensile bond strength of 60 bovine incisors that were bleached with 35% hydrogen peroxide. While they discovered a decrease in the microtensile bond strength of the teeth that were bleached with 35% hydrogen peroxide compared to the control group, this difference was not statistically significant. The microtensile bond strength means (MPa) were 9.95, 7.48, 7.46, 6.45 and 7.19 for control/no bleaching, catalase, 2% chlorhexidine gel, 1% sodium hypochlorite, and distilled water, respectively [ 36].
Another technique that has recently attracted attention is the use of lasers. Gabriel et al. showed that treatment with Er:YAG laser and total-etch adhesive procedures increased the bond strength of restorative materials to bleached dentin [ 37]. Basir et al. (2016) demonstrated that the use of Er:YAG and CO2 lasers and 10% sodium ascorbate improved the bond strength of composite resin to specimens in the at-home bleaching procedure, and the use of Er:YAG laser in the in-office bleaching procedure resulted in the highest bond strength compared to other techniques [ 38]. Additionally, Basir et al. evaluated the effect of surface preparation with Nd:YAG, Er:YAG, and CO2 lasers and sodium ascorbate on freshly bleached enamel with laser and reported that Nd:YAG laser created melted areas on the tooth surface. However, Er:YAG laser created irregularities and surface porosities, and the CO2 laser melted the surface layer of the tooth substrate and created micrometer cracks. In the present study, we found that bleached teeth prepared with Er:YAG laser had a higher tensile bond strength than the bleached control group without preparation. However, there was no significant difference between the unbleached teeth and the bleached teeth prepared with sodium ascorbate, which is consistent with previous studies.
On the other hand, sodium ascorbate did not induce any changes except for those caused by the bleaching effect. The findings suggest that surface preparation with Er:YAG, Nd:YAG, and CO2 lasers following tooth bleaching with laser beams could enhance the bond strength of resin materials to the newly bleached tooth [ 39]. Er:YAG irradiation on the tooth surface caused fusion and re-crystallization of the surface, which was proportional to the density of the laser energy used. Laser irradiation induced some morphological changes on the tooth surface by reducing the percentage of calcium and phosphorus in the tooth structure, leading to changes in the hydroxyapatite structure and its re-crystallization [ 40-42]. The re-crystallization of hydroxyapatite crystals results in better bonding to the tooth surface. Therefore, an increase in bond strength after laser irradiation might be attributed to this phenomenon. In other words, laser irradiation evaporates the free radicals generated during the bleaching procedure. Furthermore, apatite peroxide, which is formed due to the replacement of peroxide ions by hydroxyl radicals in the apatite crystals, is re-crystallized to form hydroxyapatite [ 43].
CONCLUSION
Overall, the results of this study suggest that both 10% sodium ascorbate and Er:YAG laser treatment can enhance the microtensile bond strength of composite resin to bleached enamel. Furthermore, there was no significant difference in the effect of 10% sodium ascorbate and Er:YAG laser treatment on the microtensile bond strength of composite resin to bleached enamel. Moreover, the bleaching can significantly decrease the microtensile bond strength of the enamel.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the ethical committee of Ahvaz University of Medical Sciences (IR.AJUMS.REC.1397.243).
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of this study are available from the corresponding author [N.E] upon reasonable request.
FUNDING
This study was supported by a research grant (grant number IR.AJUMS.REC.1397.243) from Ahwaz University of Medical Sciences, Iran.
ACKNOWLEDGEMENTS
Declared none.


