All published articles of this journal are available on ScienceDirect.
Effect of Pharmacological Methods in Accelerated Orthodontics: A Literature Review
Abstract
Aims:
This study aimed to perform a literature review on the effect of pharmacological methods in accelerated orthodontics and the evidence of published studies.
Materials and Methods:
A search of the reported literature till December, 2020, was conducted using “PubMed,” “Google Scholar,” and “manual search.” The MesH terms and keywords in the search criteria were “tooth movement,” “orthodontics,” “pharmacological,” and “drugs” in various combinations. The search was confined to the English language. Data extraction was done under the heading authors, country and year, study design (level of evidence), study type, and pharmacological agents. The level of evidence of included studies was in accordance with the National Health and Medical Research Council.
Results:
Five hundred forty-seven studies were retrieved from different databases, and 12 were included in this review. Out of 12 included studies, 10 were animal studies, and two were human trials. Pharmacological agents utilized for intervention were prostaglandin, followed by relaxin, vitamin D, and parathyroid hormone. The level of evidence in the human study was reported as level II, and for animal studies, level III; all the included studies recorded accelerated tooth movement. The risk of bias in the included animal studies was unclear, and the risk was low and moderate for human studies.
Conclusion:
Prostaglandin, interleukins, parathyroid hormone, and vitamin D are commonly used in published literature for accelerating tooth movement. Nonetheless, all these experimented drugs have few or other unsolicited adverse effects. Further studies with long-term follow-ups are recommended to support the utilization of pharmacological methods in accelerated orthodontic movements.
1. INTRODUCTION
Accelerating orthodontic tooth movement minimizes the duration of the treatment with fewer side effects. It also has numerous potential benefits, including differential tooth movement, the enhanced envelope of tooth movement, and better post-treatment retention. Fixed orthodontic treatment usually takes 24 to 36 months [1, 2], and the enhanced duration of treatment poses various complications that include periodontal problems, external root resorption, and patient compliance [3-5]. Orthodontists are constantly motivated toward alternative approaches to minimize the duration of the treatment [6-9].
Cunningham first proposed vertical cuts in intra-dental areas while treating palatally inclined maxillary lateral incisors [10, 11]. A localized inflammatory response will be initiated by creating a surgical wound in the bone [10, 12-15]. This procedure causes osteoclast differentiation, eventually leading to bone resorption and accelerating tooth movement. This acceleration of tooth movement might be possible in the presence of chemokines and cytokines through the prostaglandin E2 pathway and the RANK/RANKL pathway. Nevertheless, this outcome is not permanent and persists for four months [14], and a repetition of this entire procedure is required to allow accelerated tooth movement.
The need for a treatment approach is evident to minimize the duration of orthodontic treatment without affecting the outcome. Subsequently, numerous methods have been introduced to enhance tooth movement in contemporary orthodontics, including mechanical stimulation, surgical, and pharmacological methods. Among these methods, pharmacological methods in accelerated orthodontics and their effect have been evaluated only in a few published works of literature. There is a need to study reported pharmacological approaches and their impact on accelerated orthodontics. Therefore, this literature review aims to revise pharmacological methods utilized in accelerated orthodontics and produce an evidence-based report of included studies [15].
2. MATERIALS AND METHODS
As this is a literature review, PROSPERO registration was not required. However, this review has followed the PRISMA guidelines to acquire evidence-based results. The PICO question developed is “What are the effects of pharmacological methods on accelerated tooth movement in humans and animals? Whether the population is humans or animals (P), intervention is the pharmacological method (I), a comparison is not applicable (C), and the outcome is orthodontic tooth movement (O). In this review, the effects of pharmacological methods on accelerated tooth movement are discussed. However, no hypothesis has been developed.
2.1. Data Extraction
The literature was searched electronically on PubMed and Google Scholar from inception till December, 2020. In addition, a manual search was performed on peer-reviewed journals, and cross-referencing was done. Articles other than the English language were excluded. The medical subject headings [MesH] used for this review were “orthodontic patient,” OR “orthodontic therapy,” OR “orthodontic treatment,” OR “Orthodox,” OR “tooth movement” OR “teeth” AND “Pharmacological” OR “Drug,” OR “Local factor,” OR “Vitamin D,” OR “Prostaglandin,” OR “Cholecalciferol,” AND “Accelerated tooth movement,” OR “Fast treatment,” OR “treatment time “OR “rapid tooth movement.”
2.2. Eligibility Criteria
The inclusion criteria followed for this review were studies performing pharmacological interventions, such as prostaglandin, vitamin D, hormone, interleukins, and parathyroid for orthodontic tooth movement. All the included articles were in the English language. There was no restriction on the age of the patients, and animal studies were also included in this review. Exclusion criteria were studies that performed accelerated tooth movement without pharmacological intervention.
2.3. Study Selection
Evaluators independently reviewed and examined the abstract and titles of the articles. The full text was considered if the articles were potentially eligible, and the eligibility criteria were not determined by the abstract alone. Full-text articles were assessed following inclusion-exclusion criteria.
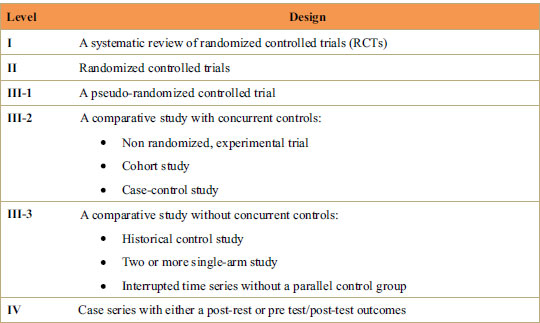
The data extraction of full-text articles was done under the following headings: authors, country, and year of publications, pharmacological intervention, study design, level of evidence, and study type (animal or human study). The level of evidence was categorized according to the National Health and Medical Research Council [16] (Fig. 1).
2.4. Risk of Bias
The quality assessment of the included animal study was performed by the SYRCLE risk of bias tool. This tool is designed to evaluate studies on animal experimental trials. There are 10 questions related to the possible risk of bias from the allocation of the sample to the publication of the study. The answer on this scale is given as “Yes,” “No,” or “Unclear.”
The assessment of studies on humans, mainly randomized control trials, was performed by the Cochrane Collaboration tool. This tool has 7 questions evaluating risks from sample sequence allowance to outcome reporting. The answer on this scale is given as: “yes,” “no,” and “unclear.”
3. RESULTS
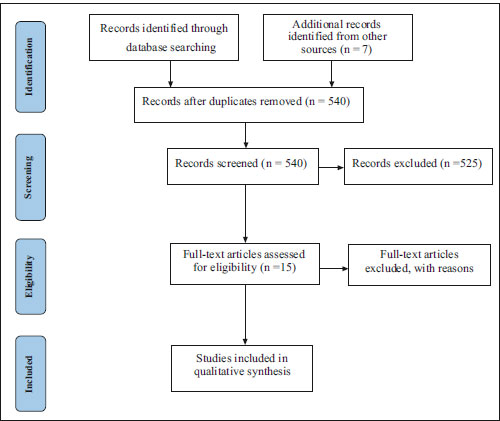
Fig. (2) illustrates the complete search of the included article with every step. A total of 540 articles were retrieved from the databases searched (533 from electronic search and 7 from manual search) (Fig. 2) on the effect of pharmacological methods on orthodontic tooth movements (Table 1). Five hundred eighteen articles were excluded after screening and duplication removal. Fifteen articles were retrieved for full-text reading, out of which three articles were excluded. Finally, 12 articles (2 studies on humans and 10 animal studies) were included in this review for data extraction [17-28].
Table 2 demonstrates different pharmacological methods utilized in the included studies. Out of 12 studies, 5 studies utilized prostaglandin (Yamasaki et al. [26], Yamasaki et al. [27], Seifi et al. [25], Yamasaki et al. [28], Kale et al. [18]), 3 studies used relaxin (Liu et al. [22], Madan et al. [23], Mc Gorray et al. [24]), 3 studies utilized Vitamin D, and 2 studies used parathyroid hormone (Soma et al. [19], Soma et al. [20],) as a pharmacological agent. The level of evidence determined in human studies was level II, signifying that studies were randomized controlled trials, and animal studies were level III, indicating non-randomized experimental trials, case-control, and cohort studies.
| Retrieved Articles and Search Combinations | n |
|---|---|
| “Tooth movement” AND “pharmacological” | 39 |
| “Tooth movement “AND “drugs” | 82 |
| “Tooth movement” AND “orthodontics” AND “pharmacological” | 23 |
| “Tooth movement” AND “orthodontics” AND “pharmacological” and “drugs” | 4 |
| “Orthodontics” AND “pharmacological” | 124 |
| “Orthodontics” AND “drugs” | 239 |
| “Orthodontics” AND “pharmacological” AND “drugs” | 15 |
| Pharmacological Agent | Studies |
|---|---|
| Parathyroid hormone | Soma et al. [19], Soma et al. [20] |
| Relaxin | Liu et al. [22], Madan et al. [23]. Mc Gorray et al. [24] |
| Prostaglandins | Yamasaki et al. [26], Yamasaki et al. [27], Seifi et al. [25], Yamasaki et al. [28], Kale et al. [18] |
| Vitamin D | Collins et al. [17], Kale et al. [18], Kawakami et al. [21] |
| Authors, Year, and Country | Pharmacological Agent | Level of Evidence | Animals | Overall Result | Did the Drug assist Accelerated Orthodontics Tooth Movement |
|---|---|---|---|---|---|
| Yamasaki et al. [26] 1980 Japan | Prostaglandins (PGs-PGE1 or PGE2) | Level III | Sprague-Dawley male rats | A dose-dependent increase in the appearance of osteoclasts when PGs were injected into the gingiva. | Yes |
| Yamasaki et al. [27] 1982 Japan | Prostaglandins | Level III | Monkey (Female Macaca fuscata) |
Local administration of PGE1 or PGE2 (40 ug/site) in gingiva increased the rate of tooth movement by 2-fold. | Yes |
|
Collins et al. [17] 1988 USA |
1,25dihydroxycholecalciferol (1,25D) | Level III | Young adult cats | Intra-ligamentous injections of a solution of 1,25D moved teeth 60% further than matched control teeth—increased numbers of mononuclear osteoclasts. | Yes |
|
Soma et al. [20] 1999 Japan |
Parathyroid hormone | Level III | Male Wistar rats | Continuous administration of PTH infusion (10 μg) doubled the rate of tooth movement. | Yes |
|
Soma et al. [19] 2000 Japan |
Parathyroid hormone | Level III | Male Wistar rats | PTH-MC injection (1 pg/400g body weight) increased tooth movement by 1.6-fold, along with active osteoclastic bone resorption and a widened periodontal space. |
Yes |
| Seifi et al. [25] 2003 Iran | Prostaglandin E2 (PGE2) | Level III | 8-week-old male Wistar rats | Significant acceleration of orthodontic tooth movement TM after PGE2 injection. | Yes |
| Kawakami et al. [21] 2004 Japan | 1,25-dihydroxy vitamin D3 (1,25(OH)2D3) | Level III | 7-weeks old male Wistar rats | Stimulated alveolar bone formation on the mesial side. Significant increase in osteoblast surface value on the tension surface. | Yes |
|
Kale et al. [18] 2004 Turkey |
prostaglandin E2 (PGE2) and 1,25-dihydroxycholecalciferol (1,25-DHCC) |
Level III | 6-week-old male Sprague- Dawley rats |
Both PGE2 and 1,25-DHCC enhanced the amount of tooth movement significantly. | Yes |
|
Madan et al. [23] 2007 USA |
Recombinant human relaxin | Level III | 45-day-old Sprague-Dawley rats | Relaxin did not stimulate significantly greater or more rapid tooth movement but reduced PDL fiber organization and increased tooth mobility at the initial stages. | No |
|
Liu et al. [22] 2005 USA |
Human Relaxin | Level III | 45 days old Sprague-Dawley male rats | Both modes of application (minipumps and subcutaneous injections) accelerated the initial stages of orthodontic tooth movement. | Yes |
table 3 and 4 demonstrate the findings of included animal and human studies. The results of this study show that enhanced alveolar bone formation, higher levels of mineral apposition rate and osteoblast surface [21], along with increased numbers of mononuclear osteoclasts [17], were found with the application of 1,25-dihydroxy vitamin D3 (1,25(OH)2D3) during tooth movement. Local application of prostaglandins (PGE1 or PGE2) resulted in a dose-dependent increase of osteoclasts on proximal aspects [26] and almost double the rate of tooth movement [25, 27]. Recombinant human relaxin either did not significantly stimulate rapid tooth movement [23] or had a mild effect [22]. 1,25-DHCC increased orthodontic tooth movement, and there was an increase in the number of Howship’s lacunae and capillaries on the pressure side [18].PTH infusion [20] and PTH-MC injection [19] almost doubled the rate of tooth movement with active osteoclastic bone resorption and a widened periodontal space in these cases. In humans, locally administered prostaglandins in gingiva near the orthodontically treated teeth caused almost double the rate of tooth movement [28], while relaxin did not significantly increase tooth movement [24].
3.1. The Quality of the Studies Included
Table 5 summarizes the risk of bias amongst the included animal studies using the SYRCLE tool [29]. Most of the studies were considered to have an unclear risk of bias, except 2 with moderate risk [18, 23]. Due to the inconsistency of available data, it was difficult to determine the risk of bias in most studies. The domains that represent an unclear risk of bias were random sequence generation, allocation concealment, and blinding outcome of the study. The low risk of bias was presented in the domain, namely random outcome assessment and blinding. No information was provided on whether investigators were blinded and animals were randomly allocated for the research. Finally, it was difficult to determine whether studies were free of any other issues that could increase the risk of bias.
| Authors, Year, and Country | Pharmacological Agent | Level of Evidence | Humans | Overall Result | Did the Drug assist Accelerated Orthodontics Tooth Movement |
|---|---|---|---|---|---|
|
Yamasaki et al. [28] 1984 Japan |
Prostaglandins (PGE1) | Level II | Adults | Local administration of PGE (10 pg per site) almost doubled the rate of tooth movement. | Yes |
|
Mc Gorray et al. [24] 2012 USA |
Relaxin | Level II | Adults | Tooth movement over the 8-week treatment period did not differ for the relaxin group and placebo. | No |
| S.No. | Animal Studies | Sequence Generation | Basic Characteristics | Allocation Concealment | Random Housing | Blinding | Random Outcome Assessment | Blinding | Incomplete Outcome Data | Selective Outcome Data | Other Sources of Bias |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Yamasaki et al. [26] 1980 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| 2 | Yamasaki et al. [27] 1982 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| 3 | Collinset al. [17] 1988 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| 4 | Soma et al. [20] 1999 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Unclear | Unclear | Unclear |
| 5 | Soma et al. [19] 2000 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Unclear | Unclear | Unclear |
| 6 | Seifi et al. [25] 2003 | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Unclear | Unclear | Unclear | Unclear |
| 7 | Kawakami et al. [21] 2004 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| 8 | Kale et al. [18] 2004 | Unclear | Yes | Unclear | Yes | Unclear | Yes | Yes | Unclear | Unclear | Unclear |
| 9 |
Liu et al. [22] 2005 |
Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Unclear | Unclear | Unclear | Unclear |
| 10 | Madan et al. [23] 2007 | Unclear | Unclear | Unclear | Unclear | Unclear | yes | Yes | yes | Unclear | Unclear |
Table 6 represents the risk of bias for included randomized controlled trials by Cochrane collaboration tools [30]. Of the 2 included studies, one study by Mc Gorry et al. [24] represents a low risk of bias, and another study by Yamasaki et al. [28] represents an unclear risk.
4. DISCUSSION
The present review established the relationship between accelerated orthodontics and pharmacological agents. The literature yielded very few studies reported in this context. Prostaglandins are paracrine lipid inflammatory mediators that act on nearby cells and encourage resorption of the bone by increasing the osteoclasts' number. In 1980, Yamasaki and colleagues [26] investigated the influence of local prostaglandin administration in rats, and later, in 1984, human clinical trials were conducted [28]. The authors concluded that prostaglandin local administration is very effective and safe in humans. Based on these animal and human studies, Yamasaki and colleagues [28] recommended that the local administration of prostaglandin effectively accelerated orthodontic tooth movement. Consequently, a systematic review by Eltimamy et al. [31] on human studies reported inconsistency in evidence supporting prostaglandin administration to accelerate tooth movement. Overall, studies have supported the use of prostaglandin to accelerate orthodontic tooth movement, yet more studies are required to support these findings.
Subsequently, Seifi and colleagues performed an animal study to investigate the importance of prostaglandins in association with calcium ions [25]. The authors found that combining calcium ions and prostaglandins will increase orthodontic tooth movement and stabilize root resorption. Human trials were not performed to support this combination, as suggested by Seifi and colleagues [25]. The role of relaxin was extensively studied in the remodeling of soft tissue rather than the remodeling of bone [32]. Relaxin is a hormone that helps during childbirth and is also present in the periodontal ligament and cranial suture. Relaxin has the cumulative effect of decreasing collagen at the pressure site and increasing it at the tension site.
Studies on the role of relaxin [22, 23] show that relaxin does not enhance tooth movement in rats; it can reduce periodontal ligament organization level, and periodontal ligament mechanical strength initially increases tooth movement. Few studies have suggested that remodeling periodontal ligament by relaxin might reduce the chance of relapse after orthodontic treatment [33]. A randomized control trial conducted by Mc Gorray et al. [24] recorded the tooth movement weekly on polyvinyl siloxane impression, suggesting no significant difference between the relaxin group and the control group. Authors opined that the low dosage of relaxin might have minimal effect on tooth movement. The role of the relaxin hormone on accelerated tooth movement is still not fully understood.
Parathyroid hormones elevate serum calcium concentration by stimulating bone resorption and up-regulating calcium reabsorption. The enzyme 25-hydroxy D3 1-alpha-hydroxylase in the kidneys forms more than 1, 25 dihydroxy vitamin D3 and increases calcium absorption in the small intestines. Soma and co-workers [20] reported that the constant administration of parathyroid hormone accelerates tooth movement. It was suggested that administering PTH locally is more effective than systemic administration. Parathyroid hormone dissolved in gel form allows a slow release of the hormone, which causes faster tooth acceleration compared to daily injecting PTH mixed in saline solution [34].
Various investigations [17, 18, 21] established 1, 25-DHCC to modulate bone turnover during orthodontic tooth movement using vitamin D in rats. Vitamin D and parathyroid hormone have similar features of calcium reabsorption. A comparative study investigating the effect of vitamin D and PGE injections on accelerating tooth movement suggested no statistical difference in both groups [18]. However, osteoblast formation was recorded more on the site where vitamin D was injected. This finding indicates that vitamin D has a direct effect on bone remodeling. The disadvantage is that vitamin D increases lactate dehydrogenase and creatine kinase enzyme levels when injected into the periodontal ligament. However, safety remains a significant concern for the clinical application of pharmacological methods in orthodontic treatment.
Orthodontic treatment is in increasing demand nowadays because of patients' interest in completing the treatment in less time and reducing the number of visits [21-28, 32, 33, 35-37]. Accelerating orthodontic techniques can help fasten the treatment [32, 33, 36]. Moreover, it has been observed that an increased tooth movement rate decreases the treatment time. Numerous pharmacological agents were introduced to accelerate orthodontic tooth movement and have achieved successful results [38]. Kouskoura and colleagues hypothesized that the drug carriers that do not spread into a larger area from the application site are induced only at areas of bone remodeling [39]. Another factor in successful orthodontic tooth movement includes treatment adjustment based on the patients' age. It has been found that early-stage cellular responses are delayed with increasing age [40].
Most of the reported studies were animal and case-control studies, and only two randomized control trials performed in humans were published on pharmacological methods used in accelerated orthodontics. Based on the National Health and Medical Research Council, all the animal studies achieved level III, while level II was reported for human trials. However, the available evidence is insufficient to support or contrary to pharmacological methods in accelerating tooth movement. Furthermore, the adverse effects of the pharmacological agents also need to be considered. This review establishes the relationship between pharmacological agents and accelerated tooth movement in orthodontics. Prostaglandin, interleukins, parathyroid hormone, and vitamin D are pharmacological aids in accelerating tooth movement. The review suggests further investigations with a large sample size to prove the use of prostaglandin, interleukins, parathyroid hormone, and vitamin D in accelerated tooth movement to reduce the usual orthodontic treatment period.
CONCLUSION
Pharmacological methods have more side effects, and most are still in the investigational phase. Prostaglandin, interleukins, parathyroid hormone, and vitamin D have been reported in the literature for accelerating tooth movement. Nonetheless, all these experimental drugs have few or other unsolicited adverse effects. Only limited human trials are available, and long-term outcomes are not clearly stated. Further studies with long-term follow-ups are recommended to support the use of pharmacological methods for accelerating orthodontic movements.
LIST OF ABBREVIATIONS
| P | = Population is human and animals |
| I | = Intervention is pharmacological method |
| C | = Comparison is not applicable |
| O | = Outcome is orthodontic tooth movement |
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data availability statement mentioned above is not applicable to this manuscript since it is a literature review. The author confirms that the data supporting the findings of this study are available within the articles mentioned in the table.
FUNDING
This study was funded by the Deanship of Scientific Research at Majmaah University in Saudi Arabia under project number (R-2023-479).
CONFLICT OF INTEREST
The author declares no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The author would like to thank the Deanship of Scientific Research at Majmaah University for supporting this work.


