All published articles of this journal are available on ScienceDirect.
Zirconium Dioxide Nanoparticles Effect on the Color Stability of Maxillofacial Silicone after Outdoor Weathering
Abstract
Background:
Maxillofacial prostheses made of silicone elastomers tend to lose color. Despite advances in materials and processes, color change over time remains a challenge.
Objective:
This in vitro study aimed to observe how zirconium dioxide (ZrO2) nanoparticles impact the color stability of M511 heat temperature vulcanizing (HTV) silicone elastomer following outdoor weathering.
Methods:
ZrO2 nanoparticles were added in concentrations of 1%, 2%, and 3% by weight to the M511 HTV silicone elastomer. Brilliant red- and mocha-pigmented silicone pigments were utilized, along with colorless silicone as a base control. A total of 90 disk-shaped specimens were fabricated and divided into nine experimental groups, each containing ten samples (n = 10). All specimens were subjected to 6 months of outdoor weathering. A colorimeter was used to measure the values of L*a*b* according to the CIELab system. The 50:50% perceptibility threshold (∆E* = 1.1) and acceptability threshold (∆E* = 3.0) were used to interpret recorded color differences. At the 0.05 level of significance, the 1-way ANOVA and the Tukey post hoc test were used in the statistical analysis.
Results:
All evaluated specimen groups experienced a chromatic alteration (∆E* > 0). The ∆E* values exceeded the perceptible threshold in all groups (1.1 units). The ∆E* value of the colorless group and the red pigment with and without ZrO2 nanoparticles were both above the acceptable threshold (p < 0.001). Mocha control was also above the acceptable level but was not statistically significant (p > 0.99). ZrO2 nanoparticles showed a reduction in color change.
Conclusion:
According to this in vitro study, all specimens underwent color changes. Even colorless silicone exhibited a significant color change. The red pigment showed a highly significant chromatic alteration. ZrO2 nanoparticles showed important protection and a reduction in color change. Its protecting action increased with an increase in the concentration of ZrO2 nanoparticles (3% ZrO2 > 2% ZrO2 > 1% ZrO2).
1. INTRODUCTION
Maxillofacial prostheses are a therapy option for people with facial deformities that cannot be restored surgically [1-3]. The use of maxillofacial prosthetic materials is rising in patients with significant face abnormalities to improve functional and esthetic shortcomings [4-7].
For almost 50 years, maxillofacial prosthetic silicone elastomeric (MFPSE) materials have been the material of choice for repairing the anatomy and esthetic functioning of craniofacial abnormalities [8-11]. However, the durability and care of silicone prostheses are of concern [12, 13].
The color of the facial prosthesis is a crucial factor for the patient to consider while evaluating the prosthesis [14-16]. Discoloration is the most common reason for replacing facial prostheses because silicone elastomer exhibits significant chromatic change with time. The color is only considered practical for approximately six to twelve months after application and must be renewed [17-20].
Sunlight, moisture, temperature, air pollution, rain, and wind are the primary causes of outdoor color change and polymer deterioration. A photo-oxidative attack, caused by the combined actions of oxygen and sunlight on the chemical structure of a material, is the precise mechanism through which deterioration occurs. The nature and severity of these adverse changes may vary based on the geographic region, climate, and environment in which the prosthesis is utilized [21-25].
Polymer outdoor performance can be simulated; in many circumstances, polymer lifetime in service can be estimated using artificial weathering. However, rapid artificial weathering may impact the degradation pathway, resulting in inaccurate estimations of polymer lifetime [26-29].
In terms of physical and optical qualities, researchers found that adding nanoparticles to polymers provided promising results. Nano-sized ZrO2 has a small size, an active function, a large specific area, and a strong interaction with organic polymers. As a result, it can increase the polymer's optical and physical properties and its resistance to environmental stress-induced breaking and aging [30, 31]. ZrO2 nanoparticles have excellent mechanical and electrical properties, a high dielectric constant, a wide band gap, and strong thermal stability. ZrO2 uses include optical applications, gas sensors, solid fuel cells, high-durability coatings, and catalytic agents [32].
The CIE Lab system, designed by the Commission Internationale de L'Eclairage (CIE), is often used to define color notations. The overall color difference attributed to all color coordinates changes is designated as ∆E*. The perceptibility threshold for light-skinned maxillofacial prosthetic silicone is 1.1, while the acceptability threshold is 3.0 [33, 34].
There are currently no in vitro or in vivo studies examining the influence of ZrO2 nanoparticles on the color stability of maxillofacial silicone following outdoor natural weathering. As a result, the current work attempted to determine the effect of ZrO2 nanoparticles on the color stability of maxillofacial silicone elastomer following outdoor weathering. According to the null hypothesis, outside weathering will not change the color of maxillofacial silicone, and the addition of nano ZrO2 will not protect the silicone from chromatic alteration.
2. MATERIALS AND METHODS
2.1. Materials
The materials used in this study were obtained from their respective manufacturers: parts A and B of M511 HTV maxillofacial silicone elastomer from Technovent Co. Ltd. (Bridgend, UK), ZrO2 nanoparticles of 99.9% purity, 20 to 30 nm, SSA >35 m2/g, density: 5.89 g/cm3, nearly spherical, white nanopowder from SkySpring Nanomaterials Inc. (Houston, TX, USA), dry pigment (Brilliant red and mocha) from Technovent Co. Ltd. (Bridgend, UK).
2.2. Outdoor Weathering
The specimens were weathered outside at Sulaimani, Kurdistan region, Iraq. From December 11, 2021, to June 11, 2022, samples were placed on the rooftop of the University of Sulaimani's College of Science, Department of Physics. Stainless steel ligature wire was used to secure specimens to a wooden rack. The samples were left uncovered and exposed to climate conditions during the weathering process. The average monthly temperature and other environmental parameters during outdoor natural weathering are listed in Table 1.
2.3. Experimental Design and Sample Preparation
A total of 90 disk-shaped specimens (2 mm thick, 20 mm diameter) [35, 36] were prepared and evenly divided throughout nine experimental groups, with ten samples (n = 10) in each group. The control specimens were fabricated without adding ZrO2 nanoparticles (0% ZrO2). However, ZrO2 nanoparticles were combined with silicone at 1%, 2%, and 3% by weight to create the study specimens. Fig. (1) displays the dispersion of these specimens by pigment (i.e., red and mocha).
| Date | Max T (C°) | Min T (C°) | Avg T (C°) |
Avg Wind Speed (Kmph) |
Rainfall (mm) |
Snowfall (mm) |
Humidity (%) |
UV Index |
Avg Sun h/days |
|---|---|---|---|---|---|---|---|---|---|
| December 2021 | 13 | 5 | 10 | 6.5 | 116.6 | 0.00 | 52 | 3 | 309/25 |
| January 2022 | 7 | 0 | 4 | 6.6 | 254.2 | 280 | 74 | 2 | 273/17 |
| February 2022 | 12 | 3 | 9 | 6.9 | 97.2 | 105 | 65 | 4 | 255/21 |
| March 2022 | 14 | 4 | 10 | 9.2 | 63.4 | 71 | 58 | 5 | 312/29 |
| April 2022 | 25 | 12 | 20 | 9 | 26.3 | 0.00 | 40 | 5 | 333/26 |
| May 2022 | 30 | 14 | 24 | 11 | 19.9 | 0.00 | 35 | 7 | 351/28 |
| June 2022 | 39 | 22 | 33 | 11.4 | 2.4 | 0.00 | 19 | 8 | 359/30 |
Data source: https://www.worldweatheronline.com/as-sulaymaniyah-weather-averages/as-sulaymaniyah/iq.aspx
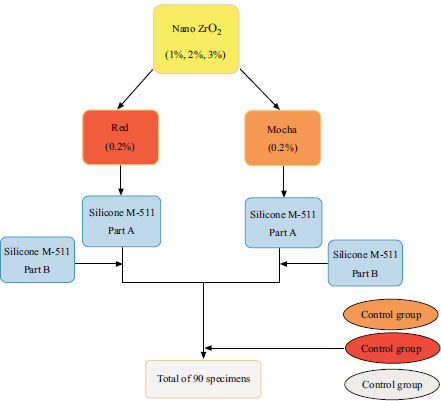
By laser-cutting cast-iron sheets, metal molds were made. The iron sheet utilized had a thickness of 2 mm, and each mold contained 16 specimen holes. Two stainless-steel plates with precise outside dimensions were cut for each mold to sandwich the mold between them and withstand clamping force.
According to the manufacturer's instructions, the M511 silicone elastomer is provided as a base (part A) and catalyst (part B), mixed in a ratio of 10:1 by weight. The weight of the pigment was equivalent to 0.2% of the silicone's overall weight [17, 35, 37-40].
First, parts A and B of the silicone were measured using a digital electronic weight balance (Nimbus® Analytical, Adam Equipment Inc., Oxford, CT, USA). Next, parts A and B of the silicone were mixed according to the manufacturer's instructions in a vacuum mixer (AX-2000C; Aixin Medical Equipment Co. Ltd., Xiqing, Tianjin, China) for 5 minutes at a speed of 360 rpm and a vacuum of -0.09 MPa. This was done to prepare M511 silicone without ZrO2 nanoparticles and pigment. The silicone specimens with each pigment were then prepared by weighing the pigment, adding it to Part A of the M511 silicone, and mixing them for 10 minutes under a vacuum. However, only mixing is done for the first 2 minutes to avoid sucking up any of the pigments with the vacuum. The study group specimens were made by first weighing the ZrO2 nanoparticles and the pigment and mixing them in the M511 silicone component A. After that, a vacuum mixer was used to combine them for 10 minutes. The vacuum was turned off for the first 2 minutes to prevent the suction of the pigment and ZrO2 nanoparticles, precisely as it had been for earlier silicone specimen preparation with only pigment content. The mixing bowl was then set aside to cool to room temperature since the mixer's rotation generated heat, reducing the material's working time. Part B was added to the vacuum mixer and mixed for another 5 minutes. After putting the mixture into the molds with a metal spatula, it was placed in a vacuum chamber for 2 minutes to remove any air bubbles that had formed during the loading process. The molds were then placed in a pressure pot (Pentola A pressione typodont; Leone S.p.A., Sesto Fiorentino, Firenze, Italy) for 2 minutes at 0.2 MPa to smooth the mixture's surface and break superficial air bubbles. Then the mold was closed and placed under a 0.03 MPa hydraulic press for 5 minutes. The molds were then sealed and clamped with G-clamps, and the material was polymerized for 1 hour in a hot air oven (Memmert; Memmert GmbH+Co KG, Schwabach, Germany).
After being removed from the molds, the specimens were washed with water and cleaned with liquid detergent before being dried with tissue paper. The specimens were then cut using a scissor to remove any excess. Samples that had obvious defects were discarded before testing. All specimens were stored in a light-proof black box to avoid potential color changes. Color measurements were performed using a digital colorimeter (WR10QC colorimeter, FRU, Longgang, Shenzhen, China). The samples were left outside to weather for six months. After outdoor weathering, specimens were cleaned with water and liquid soap and dried with tissue paper before being measured again. The CIELAB color system was used to record the values. The CIELAB system is a nearly uniform color space containing lightness coordinates, such as white-black (L), redness-greenness (a), and yellowness-blueness (b). The L, a, and b values of each specimen were measured at baseline and after six months of outdoor weathering. Color difference (∆E*) was calculated from the mean ∆L*, ∆a*, ∆b* values for each specimen using the formula below [41]:
∆E* = [(∆L)2 + (∆a)2 + (∆b)2]1/2
2.4. Statistical Analysis
Multiple 1-way ANOVAs were performed on the ∆E* values to see if there were significant color differences between the groups using statistical software IBM SPSS statistics version 24 (SPSS Inc., Chicago, IL, USA). Post hoc Tukey tests were utilized to make multiple comparisons when the 1-way ANOVA resulted in statistical significance.
3. RESULTS
The means and standard deviations of ∆E* in the control and ZrO2 nanoparticles groups for both pigments, including red and mocha, after six months of outdoor natural weathering, are presented in (Table 2 and Fig. 2). However, the ANOVA table for color alterations (∆E*) is shown in Table 3. All evaluated specimens of the control and ZrO2 nanoparticle groups experienced a chromatic alteration (∆E*>0). The ∆E* values for all groups were found to be above the perceptible threshold (∆E*=1.1). However, ∆E* was determined to be below the acceptable clinical threshold of (∆E*=3.0) for mocha groups with ZrO2 nanoparticles, demonstrating acceptable aging-dependent color changes. While; the ∆E* for the colorless silicone group, red pigment alone, and red pigment with ZrO2 nanoparticles showed highly significant color change, and all were above the acceptable threshold (∆E* =3.0).
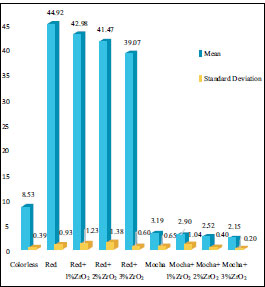
| Groups | Mean (∆E*) | SD |
|---|---|---|
| Colorless (only silicone) | 8.53b | 0.39 |
| Red | 44.92b | 0.93 |
| Red + 1% ZrO2 | 42.98b | 1.23 |
| Red + 2% ZrO2 | 41.47b | 1.38 |
| Red + 3% ZrO2 | 39.07b | 0.60 |
| Mocha | 3.19b | 0.65 |
| Mocha + 1% ZrO2 | 2.90a | 1.04 |
| Mocha + 2% ZrO2 | 2.52a | 0.40 |
| Mocha + 3% ZrO2 | 2.15a | 0.20 |
a ∆E* > 1.1 (50:50% perceptibility threshold); b ∆E* > 3.0 (50:50% acceptability threshold).
| - | SS | DF | MS | F | p-value |
|---|---|---|---|---|---|
| Between Groups | 35852.446 | 9 | 3983.605 | 6116.959 | .000* |
| Within Groups | 58.612 | 90 | .651 | - | - |
| Total | 35911.058 | 99 | - | - | - |
It is crucial to note that there were relevant variations in the ∆E* values among the groups. After six months of outdoor weathering, the colorless specimens showed a significant color alteration with respect to both perceptible and acceptable thresholds (p<0.001). For red pigment only and with ZrO2 nanoparticles 1%ZrO2, 2%ZrO2, and 3%ZrO2, they showed a highly significant color alteration, and the ∆E* was higher than the perceptible and acceptable thresholds (p<0.001). The ∆E* for them was 44.92, 42.98, 41.47, and 39.07, respectively. The addition of ZrO2 nanoparticles showed a significant reduction in color change for all concentrations (p=0.001). Regarding Mocha pigment, all groups with mocha pigment were above the perceptible threshold. According to the perceptible threshold, the mocha control group, 1%ZrO2, and 2%ZrO2 showed a significant increase, with p-values of <0.001, <0.001, and 0.006, respectively. The 3%ZrO2 group showed a statistically non-significant increase according to the perceptible threshold (p=0.115). All groups of mocha pigment with ZrO2 nanoparticles were below the acceptable threshold. However, mocha pigment only without ZrO2 nanoparticles was above the acceptable threshold but statistically non-significant (p >0.99). Adding 1%, 2%, and 3% ZrO2 nanoparticles to mocha pigment showed decreases in color change. However, they were statistically non-significant, and the p-values were 0.998, 0.702, and 0.131, respectively.
4. DISCUSSION
The findings of this in vitro study support rejecting the stated null hypothesis because outdoor weathering altered the color of specimens and caused a considerable color change in all groups, whereas ZrO2 nanoparticles provided good protection and reduced color alterations.
Loss of esthetics and poor durability have been identified as the most common issues with facial prostheses throughout several clinical studies. Patients’ most common reason for disliking their prosthesis is color fading [42]. Currently, different pigments and preblended combinations of pigments and opacifiers claim to boost prosthetic color stability.
This study's key criterion was outside weathering for six months. According to the researchers, a minimum of three months of outdoor weathering is required for observable change in maxillofacial materials [23]. It is improbable for an individual to be outside 24 hours a day. Suppose a facial prosthesis is exposed to 8 to 12 hours of outdoor exposure per day. In that case, the duration of clinical service could range from 12 to 18 months. The current study's six months of weathering included three months of cold weather (December, January, and February) and three months of hot weather (March, April, May, and half of June; weather data is shown in Table 1).
The deterioration characteristics of materials are determined by the quality and quantity of radiant energy to which the material is exposed, as well as whether the absorbed radiation contains enough energy to trigger a chemical change, resulting in material degradation [24]. The specimens in this study were left in the open without a glass cover to expose the specimen surfaces to a wide range of weathering conditions.
The color difference that the human eye can detect is referred to as the perceptibility threshold. In contrast, the color difference acceptable in esthetics is regarded as the acceptability threshold [43]. Changing a material's color in a clinical setting is permitted if the change is less than the acceptable threshold and more than the detectable threshold. This suggests that a material's color change can be clinically detected while being aesthetically pleasing. In this investigation, the color change of mocha is aesthetically acceptable. However, colorless silicone and red pigment are not aesthetically acceptable because ∆E* is well beyond the acceptable threshold.
In line with expectations, the color of additive-free silicone samples exposed to outside weathering has changed noticeably. Silicone may absorb UV light energy, causing polymer chains to break and free radicals to form. The most significant impacts on maxillofacial silicones are caused by sunlight, which contains wavelengths such as UV light, visible light, and infrared light. When maxillofacial prostheses are exposed to sunlight, the silicone absorbs photons, causing photodegradation [44-46]. Through a process known as photodegradation, molecules are fragmented, and photons permanently change their forms [47]. While most research on the influence of weathering on the properties of maxillofacial silicones has used artificial aging, there has been little research on the effect of natural weathering. Furthermore, each geographic region's natural weather has special and unique properties. The results obtained in each region would be advantageous to the inhabitants. Therefore, the tested maxillofacial silicones in this study were subjected to outdoor weathering.
The unpigmented group in the current study had a significant color change considering the perceptibility and acceptability thresholds. Hatamleh et al. reported that the unpigmented group had a considerable color change, which they attributed to continuous chemical polymerization [48]. Furthermore, these color changes may be induced by increased cross-linking generated by continuous silicone polymerization or by side reactions among impurities present in the silicone [23, 44, 49, 50]. A study by Polyzois [24] on the color stability of non-pigmented silicone elastomers following outdoor weathering discovered that silicone elastomers after outdoor weathering for one year resulted in visually noticeable color change. The exposure period and the silicone elastomer influenced color stability [42]. Our findings support previous research that showed colorless silicone prone to chromatic change [23, 51-53]. The red pigment showed a significant color change regarding both perceptibility and acceptability threshold (P<0.001). Red was chosen as the intrinsic color for this study based on the findings of Kiat-Amnuay et al. The red pigment was shown to have the most substantial negative impact on the color stability of silicone elastomers in their study [54]. Beatty et al. [49] studied the effect of UV light exposure on the color of dry-pigmented maxillofacial elastomer. They found that red cosmetic dry earth pigment underwent significant color changes after 400 hours of exposure. Another study discovered that the significant color change observed is primarily due to the loss of red pigment caused by irradiation lighting [48]. Colorants and polymers are commonly harmed by ultraviolet light. This electromagnetic wave covers only a small portion of the visible spectrum. Many researchers have used artificial weathering or aging chambers to simulate the outdoor environment, which helps evaluate the color deterioration of materials [42, 51, 54-56]. In contrast, it has been claimed that accelerated aging causes more color changes than natural aging [57, 58]. The natural outdoor weathering process may more effectively mimic natural environmental conditions. However, the limited application of natural aging could be that the procedure is time-demanding, and there is a lack of standardization based on climate conditions in different geographic regions.
According to the results of this study following outdoor weathering, the control group (containing only pigments) showed the most significant color difference of all the groups. This is because elastomers have the inherent tendency to lose color with weathering.
According to Haug et al. [23] who evaluated the color stability of a commonly used colorant-elastomer combination after exposure to weathering, several of the combinations exhibited color changes due to coloring. In a previous study investigating the effect of nano-opacifiers (TiO2 and ZnO nanoparticles) on the color stability of M511 maxillofacial silicone after outdoor weathering, they found that incorporation of nano-oxides improved the color stability of M511 maxillofacial silicone elastomer and also acted as an opacifier. Specimens with ZnO nanoparticles showed minimal or no color change after outdoor weathering [59]. Another study examined the effect of SiO2 nanoparticles on the color stability of M51 maxillofacial silicone and found that the light transmission of all experimental groups decreased significantly [60]. Another study evaluated the impact of TiO2, ZrO2, and silica nanoparticles on the color stability of pigmented polydimethylsiloxane after UVB storage. They found that polydimethylsiloxane elastomers loaded with 1% TiO2 demonstrated less color change compared to ZrO2 and silica nanoparticles [61].
While the color shift was reduced in the groups that contained ZrO2 nanoparticles, this protective function of ZrO2 nanoparticles increased with concentration. When ultraviolet sunlight hits nanoparticles in a medium, the electrons are made to vibrate. Since nanoparticles are smaller than the wavelengths of UV light, some of the light is simultaneously scattered and absorbed [55, 62]. In accordance with these fundamental principles, UV protection results through nanoparticle absorption and scattering. Due to the fact that ZrO2 nanoparticles scatter and absorb UV light, they offer comparable UV protection.
Another explanation behind the protecting role of zirconium dioxide nanoparticles may be due to its higher specific heat, which may allow more heat transmission to the polymer, possibly resulting in more polymerization during the curing process and thereby reducing post-curing polymerization. Post-curing polymerization may have produced color changes. The refractive index of zirconium dioxide nanoparticles is about (n= ~2.2), while maxillofacial silicone is around (n= ~1.4). Generally, light is bent more, travels shorter paths, and does not penetrate as deeply in materials with higher refractive indices. Therefore, samples containing zirconium dioxide nanoparticles are more efficient at scattering light than the other groups without zirconium dioxide nanoparticles [61, 63].
In this study, ZrO2 nanoparticles and silicone pigments were evenly dispersed in the silicone elastomer matrix using a vacuum mixer, significantly reducing silicone color change. The smaller the nano-oxide particles, the greater the UV protection may be accomplished. Therefore, it may be assumed that the ZrO2 nanoparticles (20-30 nm) utilized in this work could help materials retain their color. The groups containing ZrO2 nanoparticles had a lighter hue than the control group, as observed visually. This is because ZrO2 nano-oxides function as opacifiers [64].
This in vitro study had several limitations. In this study, the effect of natural outdoor weathering on color stability was examined. Only one type of maxillofacial silicone was tested in this study. Two pigments and a specific concentration of ZrO2 nanoparticles were tested, which could be considered a limitation. Future research should examine the effects of other variables, such as disinfectant solutions, different types of nanoparticles, silicone, and pigments.
CONCLUSION
In this in vitro study, all specimens exhibited color changes (∆E*>0) after outdoor weathering. Colorless silicone samples exhibited a considerable color change. Red pigments with and without ZrO2 nanoparticles showed significant changes (p<0.001). Mocha pigments with ZrO2 nanoparticles exceed the perceptible threshold yet are aesthetically acceptable. ZrO2 nanoparticles played an essential role in protecting the silicone samples and decreasing color change.
AUTHORS’ CONTRIBUTION
Mohammed Abdalqadir and Bruska Azhdar contributed equally to this work.
LIST OF ABBREVIATIONS
| ZrO2 | = Zirconium dioxide |
| HIV | = Heat temperature vulcanizing |
| MFPSE | = Maxillofacial prosthetic silicone elastomeric |
| CIE | = Commission Internationale de L'Eclairage |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used in this research.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
All data generated or analyzed during this study are included in the article.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGEMENTS
The authors are grateful to the Nanotechnology Research Laboratory, Department of Physics, University of Sulaimani, for laboratory support.


