All published articles of this journal are available on ScienceDirect.
Enhancing Periodontitis Treatment: A Comprehensive Literature Review of Locally Delivered Antibiotics as an Adjunctive Therapy
Abstract
Background:
Periodontitis is a prevalent and persistent infection that can significantly affect overall health. While scaling and root planing are effective treatments, they may not fully remove bacterial biofilms in difficult-to-reach areas, such as furcations and deep pockets. Therefore, numerous studies have demonstrated that supplementing mechanical debridement with locally delivered antibiotics can improve clinical outcomes.
Objective:
This study aims to review the effectiveness of different types of locally delivered antibiotics as adjunctive therapy to scaling and root planing in treating periodontitis.
Methods:
Pubmed, Scopus and Google Scholar were utilized to obtain papers addressing locally delivered antibiotics in periodontitis patients, antibiotics in periodontitis, or antimicrobial agents in periodontitis up to February 1, 2023.
Results:
Treatment with locally delivered antibiotics led to notable improvements in clinical outcomes. These improvements encompassed reductions in probing depths, gingival inflammation, and bleeding on probing. Additionally, enhancements in clinical attachment level and periodontal pocket depth reduction were consistently observed in the included studies.
Conclusion:
Based on individual antimicrobial agent analyses, we are unable to determine which local antibiotic is the best. It is challenging to accurately assess the use of sustained or released local antimicrobials due to the unique features of each product. Local medication administration into the periodontal pocket could be an effective therapy adjunct to mechanical instruments.
1. INTRODUCTION
Periodontitis is a chronic biofilm-induced inflammatory condition that is collectively a multifaceted disease. Significant contributing mechanisms occur simultaneously and combine with one another, leading to the destruction of the periodontal apparatus [1-3]. Understanding the pathogenic role of bacteria that build up in the periodontal space is essential for both preventing and treating this condition [4]. The bulk of periodontal therapeutic interventions focuses on mechanical plaque control, termed “scaling and root planing.” Mechanical periodontal treatment alone may not always be beneficial due to the complexity and difficulty of completely removing subgingival plaque and calculus, particularly at deep pockets [5, 6] and furcation defects [7].Given the limitations of mechanical debridement, treatment regimens employing antibiotics and antiseptics, as well as antibacterial agents, may be more effective than mechanical debridement alone. Systemic antibiotics, such as amoxicillin (with or without clavulanic acid) [8-10], azithromycin [11, 12], clindamycin [13], doxycycline [14], metronidazole [9, 13], tetracycline [15], and certain combinations of these are some of the therapy options [14, 16, 17].
Numerous investigations have suggested that periodontitis patients can benefit from receiving systemic antibiotics [18-21]. While others [22, 23] suggest that cautious use of systematic antibiotics should be the standard treatment due to their widespread overuse and the ensuing global development of drug-resistant bacteria, systemic antibiotics may be needed to treat severe infections that are spreading some severe forms of periodontitis, and necrotizing periodontal diseases [24].
In comparison to pharmaceuticals administered systemically, the main benefits of local treatment include fewer side effects, improved compliance, and a lower risk of bacterial drug tolerance [25].
The aim of this study was to review the efficacy and clinical outcomes of locally delivered antibiotics as adjunctive therapy to nonsurgical periodontal treatment in patients with periodontitis.
Overall, the novelty of this study lies in its exploration of the benefits and potential of locally delivered antibiotics as adjunctive therapy for periodontitis, providing valuable insights into a potentially effective and safer approach to treating this multifactorial disease.
2. METHODOLOGY
A comprehensive search was conducted to identify relevant studies addressing the use of locally delivered antibiotics in periodontitis patients. The databases PubMed, Scopus and Google Scholar were utilized for the literature search. The search was conducted up to February 1, 2023.
The following search terms and their combinations were used to maximize the retrieval of relevant articles: “locally delivered antibiotics,” “periodontitis patients,” “antibiotics in periodontitis,” and “antimicrobial agents in periodontitis.” These terms were adapted and tailored to the specific requirements and syntax of each database.
In PubMed, the search strategy employed the following syntax: (“locally delivered antibiotics” OR “local antibiotic delivery” OR “intrapocket antibiotics”) AND (“periodontitis” OR “periodontal disease” OR “periodontal inflammation”) AND (“antibiotics” OR “antimicrobial agents”). Filters for publication date were applied to retrieve articles published up to February 1, 2023.
In Scopus and Google Scholar, similar keyword-based searches were performed. The search terms were entered into the search boxes of each respective database's advanced search interface.
Following the initial search, duplicates were removed using the built-in functions provided by the databases. The titles and abstracts of the remaining articles were screened to assess their relevance to the topic. Full-text articles were retrieved for further evaluation if they met the inclusion criteria.
The inclusion criteria for study selection were as follows: (1) studies investigating the use of locally delivered antibiotics in periodontitis patients, (2) studies assessing the efficacy or effectiveness of locally delivered antibiotics as adjunctive therapy to scaling and root planing, (3) studies reporting on clinical outcomes, microbiological changes, or patient-reported measures, and (4) articles published in English.
Two independent reviewers conducted the article selection process. Any disagreements were resolved through discussion and consensus.
3. LOCALLY DELIVERED ANTIBIOTICS
Even though periodontal diseases inflict damage in a site-specific manner, localized treatment has received a lot of attention. For localized adjunctive pharmacological periodontal therapy, there are three main approaches: subgingival irrigation, mouth rinse (toothpaste or varnish), and periodontal administration of local delivery antimicrobial agents.
Subgingival irrigation, the drawbacks of washing, irrigating, and other comparable medication delivery methods result in fast outflow that leads to the insufficient exposition of subgingival biofilms to the therapy [26].
Mouth rinses (toothpaste or varnish) are helpful for reducing supragingival bacteria, reducing gum inflammation, and possibly recolonizing the subgingival ecosystem after periodontal therapy. Their primary shortcoming is the lack of accessibility to the subgingival area and deep pockets, which prevent them from acting at the intended site [27].
Therapeutic approaches (locally delivered antimicrobial agents) that are used locally should meet three requirements: they have to go to the desired area of action and reach the base of the pocket, maintain the antimicrobial agent there for a long enough period, and keep a sufficient level of concentration [28]. Different locally delivered devices and varying types of pharmaceutical drugs were used: tetracycline, minocycline, doxycycline, metronidazole, azithromycin, and chlorhexidine were checked in different delivery systems [29].
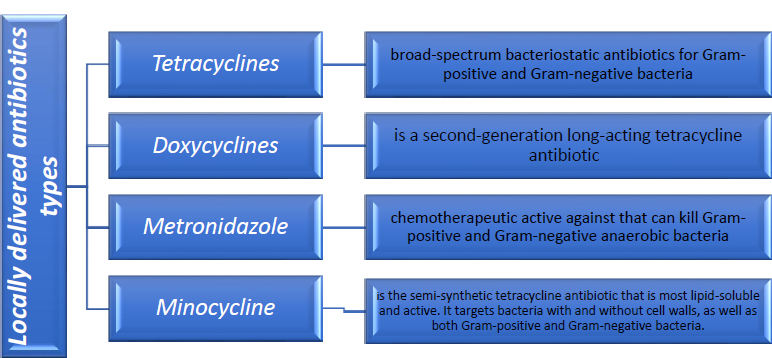
4. TYPES OF LOCALLY DELIVERED ANTIBIOTICS
As seen in Fig. (1), various types of locally administered antibiotics were employed in conjunction with non-surgical periodontal therapy.
4.1. Metronidazole
Metronidazole is a synthetic nitroimidazole derivative with antibacterial and anti-inflammatory properties [30]. Stoltze investigated metronidazole gel in 1992 and concluded that a concentration of 25% metronidazole benzoate is the appropriate concentration that could be used in the periodontal pocket [31]. In 1992, Ainamo J. et al. examined how long metronidazole would remain detectable in periodontal pockets following a single application of a novel biodegradable 25% gel and showed that a clinical impact could be produced through local antibiotic therapy (metronidazole 25% gel) that lasted for at least 24 weeks and was comparable to that achieved by standard treatment (subgingival scaling) [32]. In locations where scaling and root planing had failed, in 1999, Kinane D. compared the effectiveness of three different local periodontal antibacterial substances (25% tetracycline fibers, 2% minocycline gel, and 25% metronidazole gel) and found that the three locally applied antimicrobial methods have some advantages over scaling and root planing alone [33]. In 1992, Klinge et al. compared the clinical efficacy of scaling with the use of 3 different topical metronidazole preparations and dose frequencies in the management of adult periodontitis. They concluded that subgingival scaling combined with metronidazole gel (25%) improved the clinical outcomes in the treatment of adult periodontitis, and this could be the treatment of choice [34]. Stelzel in 1996, evaluated subgingival scaling with the topical administration of a 25% metronidazole gel and found that the clinical improvement was insignificant [35]. Marmara et al., in 1997, found that, regarding achieving improvements in both clinical and microbiological aspects, local metronidazole, in conjunction with scaling and root planing, seems to be more efficient [36]. The effectiveness of ElyzolA metronidazole dental gel as a supplement to subgingival debridement (SRP with the gel) and subgingival debridement alone (SRP) was compared by Griffiths et al. in 2000, and it was discovered that combined treatment with subgingival scaling and metronidazole 25% gel was more effective than SRP alone [37]. Lie et al. compared subgingival scaling and root planing alone (SRP), subgingival SRP with the application of a 25% metronidazole gel, and subgingival SRP with the application of a 3% tetracycline ointment and reported similar results in the three different treatment regimes [38]. In pockets with recurrent inflammation, we determined whether the subgingival deposition of a 25% slow-release metronidazole dental gel was compared with SRP alone by Leiknes et al. in 2007. We showed that a 25% metronidazole gel applied locally does not improve the success rate of treatment over SRP alone in places where there is recurrent chronic inflammation [39]. Stelzel et al., in 2000, compared the effects of two applications of 25% metronidazole dental gel with subgingival scaling and root planing to the effects of scaling and root planing alone and found that there are very slight advantages over scaling and root planing alone [40]. David Herrera et al. conducted a systematic review in 2020 that included five studies and concluded that there are no statistically significant improvements [41]. The characteristics of the studies are summarized in Table 1.
| References | Study Type | Antibiotic Type | Periodontal Parameters | Time of Observation | Outcomes |
|---|---|---|---|---|---|
| Griffiths et al. (2000) [37] | RCT | Elyzol | Probing depth, clinical attachment level, and bleeding on probing | 9 months | The combination of SRP and gel was better than the standard treatment of SRP alone, and this difference lasted for nine months. |
| Lie et al. (1998) [38] | RCT | Elyzol /tetracycline | Probing depth, Relative attachment level, Bleeding on probing, Microbiological Recordings | 6 months | Showed that, as compared to subgingival scaling and root planing alone, topical treatment had modest and comparable clinical and microbiological augmentative effects. |
| Leiknes et al. (2007) [39] | RCT | Elyzol | Probing depth, clinical attachment level, bleeding on probing | 6 months | In areas with recurrent chronic inflammation, local application of a 25% metronidazole gel did not increase the success of treatment over SRP alone. |
| Stelzel and Florès-de-Jacoby (2000) [40] | RCT | Elyzol | Probing depth, clinical attachment level, bleeding on probing | 9 months | Using a metronidazole 25% dental gel as a supplementary therapy to subgingival scaling has only marginal benefits. |
The most common way to give metronidazole is in the form of Elyzol Dental Gel, which is put into the pocket with a syringe that thickens up afterward. Clinical improvements from using metronidazole gel in addition to scaling and root planing are still questionable; we need more investigations and larger sample sizes for a longer time to be better identified.
4.2. Tetracycline
In 1983, six types of biocompatible polymers filled with tetracycline were made into fibers and tested to make controlled delivery devices for periodontal therapy that would release the medicine over a number of days by Goodson et al. They suggested that tetracycline-loaded ethylene vinyl acetate monolithic fibers have properties that could be used to build a system for treating periodontal disease with tetracycline. These fibers were placed in the periodontal pocket and remained there for 7–12 days [42]. In 1995, Michalowicz et al. compared tetracycline fibers to scaling and root planing and found that tetracycline fibers improved all measured outcome measures as much as scaling and root planing did [43]. In addition, it has also been shown that tetracycline fiber therapy works better when used with scaling and root planing [44-46]. Tonetti M. et al. found that scaling and root planing combined with tetracycline fiber was far superior to root planing alone for maintenance patients who did not respond to standard treatment of furcation defects [47]. In 1996, Kinane et al. examined the effectiveness of three local delivery systems in the treatment of areas with persistent periodontal lesions in conjunction with scaling and root planing and discovered that the most significant decrease in probing depth occurred six months following treatment when scaling and root planing were combined with the application of tetracycline fibers [44]. In 2002, Friesen et al. compared the efficacy of tetracycline strips used singly or in multiples along with SRP to that of SRP alone and reported that tetracycline local administration is superior to root planing alone in decreasing probing depth, and many strips were preferable to a single strip in decreasing bleeding upon probing [48]. In areas with persisting periodontal lesions, Flemmig et al., in 1996, investigated whether supplementary topical application of tetracycline HCl in patients undergoing SPT would be beneficial for areas with persistent or recurrent periodontitis and revealed that topical use of tetracycline HCI can enhance gingival health and may lower the risk for disease development in localized persistent or recurrent periodontitis [49]. These findings are consistent with the research conducted by Newman et al. in 1994 to assess the effectiveness of tetracycline fiber therapy used adjunctively with Scaling and root planing in individuals with locally recurrent Periodontitis who were in the maintenance phase [50]. Aimetti et al. assessed the clinical, radiological, and microbiological response to local tetracycline (TE) administration with scaling and root planing, and they conlcuded that scaling and root planning with supplementary TE fibers in non-responsive areas had clinical, radiological, and microbiological advantages [51]. In 2004, Rodrigues et al. analyzed the subgingival microbiota of people with periodontitis who had either systemic or local tetracycline therapy along with scaling and root planing (SRP). They found that tetracycline fiber therapy considerably reduced the prevalence of resistant A. actinomycetemcomitans species when compared to systemic tetracycline therapy, and they suggested that using antibiotics (locally or systemically) should be done with caution and confined to individuals with more aggressive diseases or those who do not respond to conventional therapy [52]. In 2012, Goodson et al. studied scaling and root planing (SRP) in conjunction with periodontal surgery, local antibiotic therapy, and/or systemic antibiotics, and they discovered that compared to the results of SRP alone, patients who got additional therapy typically displayed improved CAL gain and/or PPD reduction [53].
Actisite periodontal fiber is the tetracycline-releasing device that has undergone the most rigorous testing. The primary analysis (Herrera et al., 2020) looked at seven studies that used Actisite and found a statistically significant added benefit. In the primary analysis, one trial of the 3% tetracycline ointment, aureomycin-which examined changes in pocket depth after 6-9 months-was included.; however, according to the systematic review, it did not provide statistically significant additional advantages (Herrera et al. 2020).
Tetracycline strip Tetracycline hydrochloride is encapsulated in ethylene vinyl acetate copolymer strips. The primary analysis (changes in periodontal pocket depth after 6–9 months) of the reference systematic review (Herrera et al., 2020) only looked at one trial, which showed an extra reduction. However, more research is required with a long maintenance period [41]. The studies’ characteristics are summarized in Table 2.
| References | Study Type | Antibiotic Type | Periodontal Parameters | Time of Observation | Outcomes |
|---|---|---|---|---|---|
| Aimetti et al. (2004) [51] | RCT | Actisite | Probing depth, clinical attachment level, bleeding on probing | 12 months | Scaling and root planing at non-responsive sites with additional Tetracycline fibers had favorable clinical, radiological, and microbiological outcomes. |
| Flemmig et al. (1996) [49] | RCT | Actisite | Plaque index, Gingival index, Gingival recession, Pocket probing depth, Bleeding on probing, Furcation invasion, Probing attachment loss, polymorphonuclear leukocyte elastase- a'-proteinase inhibitor assay | 6 months | Tetracycline HCI may improve gingival health and reduce the probability of localized, persistent, or recurring periodontitis progression. |
| Friesen et al. (2002) [48] | RCT | Tetracycline strips | Plaque index, gingival index, clinical attachment level, probing depth, bleeding on probing, gingival crevicular fluid volume, gingival crevicular fluid concentrations of tetracycline | 6 months | The use of locally administered tetracycline is preferable to SRP alone in lowering probing depth, and multiple strips that fill the periodontal pocket are superior to a single strip in minimizing BOP. |
| Goodson et al. (2012) [53] | RCT | Tetracycline fibers | Clinical attachment level, probing pocket depth | 24 months | When compared to the results of SRP alone, patients receiving additional therapy typically showed improved CAL gain and/or PPD reduction. |
| Newman et al. (1994) [50] | RCT | Tetracycline fibers | Probing depth, bleeding on probing, clinical attachment level | 6 months | Tetracycline fiber therapy enhances the efficiency of SRP in the maintenance phase of the management of localized recurrent periodontitis sites. |
| Wilson et al. (1997) [54] | RCT | Tetracycline fibers | Probing depth, Recession, Attachment level | 60 months | no discernible benefit was offered by tetracycline fiber use in terms of clinical attachment gain or a reduction in probing depth. |
| Tonetti et al. (1998) [47] | RCT | Tetracycline fibers | Probing pocket depth, clinical attachment levels, bleeding on probing | 6 months | For patients with furcation defects who did not respond to routine treatment, scaling, and root planing combined with tetracycline fiber were preferable to root planing alone. |
| Kinane and Radvar (1999) [44] | Actisite | Probing depth, attachment level, Gingival Index, plaque index | 6 months | The most significant decrease in probing depth was seen six months after therapy with scaling and root planning combined with tetracycline fiber application. |
| References | Study Type | Antibiotic Type | Periodontal Parameters | Time of Observation | Outcomes |
|---|---|---|---|---|---|
| Cortelli et al. (2006) [55] | RCT | Minocycline | Probing depth, plaque index, gingival index | 24 months | Both treatments decreased the average pocket depth from 90 to 360 days, while SRP, in combination with subgingival minocycline exhibited a greater decline at 270 and 360 days after treatment. |
| Killeen et al. (2016) [56] | RCT | Minocycline microspheres | Probing depth, bleeding on probing, clinical attachment level, f-interleukin (IL)- 1B/IL-1 receptor antagonist (ra) | 12 months | no extra advantage over SRP alone with the use of Minocycline microspheres. |
| Tabenski et al. (2017) [57] | RCT | Minocycline microspheres | Probing depth, bleeding on probing, clinical attachment level, Microbiological evaluation | 12 months | When compared to SRP alone, neither the use of antimicrobial photodynamic treatment nor the addition of Minocycline microspheres significantly increased the clinical and microbiological healing outcomes in deep periodontal pockets. |
| Williams et al. (2001) [58] | RCT | Minocycline microspheres | Probing depth, bleeding on probing, clinical attachment level | 9 months | Scaling and root planing combined with minocycline microspheres are more efficient than scaling and root planing alone. |
4.3. Minocycline
Dentomycin is a 5 g microcapsule gel and contains 2% minocycline hydrochloride dihydrate. Two trials were utilized in the primary analysis (PPD alterations after 6–9 months) of the reference systematic review (Herrera et al. 2020); however, no statistically significant additional benefits were evident. Arestin is composed of 1 mg of minocycline microencapsulated in poly. In 2006, Cortelli et al. analyzed the clinical effects of giving minocycline locally and performing scaling and root planing on patients with advanced chronic periodontitis. They reported that SRP plus subgingival minocycline usage resulted in a greater reduction at 270 and 360 days [55]. Killeen et al., in 2016, examined the impact of scaling and root planing in addition to minocycline microspheres in the residual pockets and found that there was no enormous advantage in using minocycline microspheres over scaling and root planing alone [56]. In 2017, Tabenski et al. looked at how adding an antimicrobial photodynamic treatment or local minocycline microspheres affected the clinical and microbial healing of deep periodontal pockets. In comparison to scaling and root planing alone, neither the antimicrobial photodynamic treatment nor the minocycline microspheres demonstrated a significant extra impact on the clinical and microbiological healing outcomes in the deep periodontal pocket [57]. Williams et al., in 2001, proposed that the effectiveness and safety of locally administered microencapsulated minocycline, scaling, and root planing combined with minocycline microspheres are superior to scaling and root planing alone at lowering periodontitis patients' probing depths [58]. The primary analysis (periodontal pocket depth changes after 6-9 months) of the reference systematic review (Herrera et al., 2020) included six studies, which showed a further decrease [41]. The studies’ characteristics are summarized in Table 3.
4.4. Doxycycline
Atridox, a two-syringe mixing system, 10% doxycycline hyclate, and a biodegradable liquid polymer gel. In 2013, a study was conducted by Ahamed et al. to determine how well-controlled local drug delivery of doxycycline works as a supplement to SRP for treating chronic periodontitis. It was found that combining SRP with 10% Atridox gel led to better results [59]. Bogren et al., in 2008, analyzed the long-term clinical and microbial effects of mechanical debridement during SPT with controlled-release doxycycline given locally, and it was concluded that locally administered doxycycline was found to have short-term effects on clinical parameters, but annual administrations did not have any additional clinical or microbiological effects on patients in the supportive phase beyond what was seen with scaling and root planing alone [60]. Dannewitz et al., in 2009, studied the effect of putting doxycycline gel along with scaling and root planing (SRP) at sites of furcation during supportive periodontal treatment (SPT), reporting that doxycycline was used once subgingivally in conjunction with SRP and temporarily reduced furcation involvement. It did not, however, lessen the frequency of reinstrumentation at locations of furcation for up to a year [61]. Eickholz et al., in 2002, assessed the clinical impact of doxycycline topical administration as an additional therapy for non-surgical periodontal treatment and found that 15% doxycycline gel with scaling and root planning, compared to SRP alone, offered a clinically relevant and more favorable PPD decrease and a statistically considerably greater relative attachment level increase [62]. In 2005, Ratka-krüger et al. investigated how the application of a biodegradable 14% doxycycline gel affected microbiological results in relation to scaling and root planing and found that at three months, there was a noticeable decrease in periodontal pathogens, thanks to the inclusion of subgingival instillation with a 14% doxycycline gel. Furthermre, the findings stabilized up to six months after therapy. No doxycycline resistance was induced [63]. In 2008, the addition of locally administered controlled-release doxycycline was studied to assess if it improved the reinstrumentation of pathologic pockets that persisted after initial periodontal therapy by Tomasi et al., who reported that locally delivered doxycycline had no effect on improving the healing outcome [64]. They also examined the clinical results of non-surgical retreatment at the site of molar furcation using ultrasonic debridement with or without the addition of locally administered doxycycline and reported that adjunctive, locally applied doxycycline did not improve the improvement in molar furcation involvement following non-surgical periodontal therapy [65]. In 2012, Tonetti et al. investigated how well slow-release doxycycline gel (SRD) worked when given to people with recurrent or persistent periodontitis alongside non-surgical treatment and discovered that SRD might provide a short-term advantage in reducing inflammation and deep pockets in periodontal patients [66]. The primary analysis (changes in PPD after 6–9 months), according to the systematic review (Herrera et al., 2020), included two trials, which suggests a statistically significant extra benefit. The efficacy of doxycycline polymers to improve periodontal health when used in conjunction with root planing is yet unknown. However, it is possible that removing calculus and dismantling biofilms before local drug delivery can improve outcomes. The studies’ characteristics are summarized in Table 4.
| References | Study Type | Antibiotic Type | Periodontal Parameters | Time of Observation | Outcomes |
|---|---|---|---|---|---|
| Ahamed et al. (2013) [59] | RCT | Atridox | Plaque index, gingival index, gingival bleeding index, microbial analysis, probing pocket depth, and Clinical attachment level. | 6 months | When SRP was used with 10% DH gel, the results were better. This shows that this therapy can be an important addition to SRP for treating chronic periodontitis. |
| Bogren et al. (2008) [60] | RCT | Doxycycline gel | Plaque index, probing depth, bleeding on probing, relative attachment level, | 36 months | Although the combined use of locally administered controlled-release doxycycline and periodontal maintenance patients showed short-term (3 months) positive effects on clinical parameters, repeated application once annually had no long-term clinical and microbiologic effects beyond those seen with subgingival mechanical debridement. |
| Dannewitz et al. (2009) [61] | RCT | Doxycycline gel | Probing depth, bleeding on probing, Vertical probing, attachment level, furcation involvement | 12 months | SRP with subgingival doxycycline has a modest short-term impact on furcation participation. The frequency of the requirement for reinstrumentation at furcation sites during SPT does not, however, decrease when it is used just once at baseline for 12 months. |
| Eickholz et al. (2002) [62] | RCT | 15% doxycycline gel (ligosan) | Probing depth, relative attachment level, microbiological examination, plaque index, gingival index | 6 months | Discovered ligosan with scaling root planing provided a statistically significantly higher relative attachment level increase compared to SRP alone and a clinically significant and more beneficial PPD decrease. |
| Ratka-Krüger et al. (2005) [63] | RCT | 14% doxycycline gel | Gingival index, plaque index, probing depth, vertical relative attachment levels, | 6 months | Adjunctive topical use of a 14% doxycycline gel significantly decreased periodontal infection without producing any discernible resistance patterns. |
| Tomasi et al. (2008) [64] | RCT | 8.8% doxycycline gel | Plaque index, probing depth, bleeding on probing, relative attachment level | 9 months | Locally delivered doxycycline had no impact on improving the healing outcome. |
| Tomasi and Wennstrom (2011) [65] | RCT | 8.8% doxycycline gel | Probing depth, bleeding on probing, relative attachment level | 12 months | Adjunctive, locally applied doxycycline did not enhance molar furcation involvement after non-surgical periodontal treatment. |
| Tonetti et al. (2012) [66] | RCT | Slow-release doxycycline gel | Probing depth, bleeding on probing, probing attachment level | 12 months | Topical doxycycline use may be beneficial in reducing inflammation and deep pockets in the short term. |
Ligosan is a 15% doxycycline hyclate in a polyethylene glycol ide/glycolide copolymer gel. The primary analysis (periodontal pocket depth changes after 6–9 months) of the reference systematic review (Herrera et al., 2020) looked at three trials. The results showed a statistically significant additional benefit [41].
4.5. Piperacillin and Sodium Tazobactam (Periofilm)
In 2013, Lauenstein et al. evaluated whether adding piperacillin/tazobactam to the treatment regimen improved clinical and microbiological outcomes and found that nonsurgical debridement with or without local antibiotic application both improved clinical periodontal results at week 26 in the same way [67]. The main analysis (PPD changes after 6–9 months) of the reference systematic review (Herrera et al., 2020) only looked at one trial, and there were no other benefits [41].
5. CLINICAL INDICATIONS
5.1. Residual Pockets
In 2002, Eickholz et al. conducted a study on patients with untreated or recurrent moderate to severe periodontitis to assess the clinical impact of topical doxycycline administration in addition to non-surgical periodontal therapy. They also concluded that adjunctive topical subgingival application of biodegradable 15% doxycycline gel was safe and offered better relative attachment level, gain, and probing pocket depth decrease than scaling and root planing alone. This means that the threshold for surgical periodontal therapy could be lowered by using topical doxycycline [62].
5.2. Furcation Involvement
Tomasi et al. analyzed the clinical results of using ultrasonic debridement with or without a local application of doxycycline to treat molar furcation sites without surgery. They concluded that adding doxycycline that was given locally did not help molar furcation involvement get better after non-surgical periodontal therapy and the advantages did not last in these challenging anatomical regions over the long run [65].
5.3. Smokers
Paquette et al., in 2003, examined how well smokers with chronic periodontitis work when given 1 mg of minocycline hydrochloride microencapsulated in 3 mg of the resorbable polymer as an add-on to scaling and root planing (SRP). The study found that treating smokers with periodontitis with SRP plus locally applied minocycline microspheres is more effective in reducing pocket depths than SRP alone [68].
5.4. Patients with Diabetes and Periodontitis
Agarwal et al., in 2017, conducted a study to look at the effects of subgingival delivery of azithromycin (AZM) (0.5% concentration) in combination with scaling and root planing (SRP) for the treatment of chronic periodontitis in people with type 2 diabetes and they concluded that the additional use of 0.5% AZM as a controlled medication delivery system improved the clinical outcome [69].
6. RESULTS
This review of literature on locally delivered antibiotics as adjunctive therapy to nonsurgical periodontal treatment in patients with periodontitis revealed several key findings. Overall, the included studies consistently demonstrated that the use of locally delivered antibiotics alongside scaling and root planing provided additional benefits in the treatment of periodontitis.
Firstly, the reviewed studies consistently reported improved clinical outcomes with the use of locally delivered antibiotics. These included reductions in probing pocket depth, clinical attachment loss, and gingival inflammation. The adjunctive use of antibiotics resulted in more effective control of bacterial biofilms in periodontal pockets, leading to improved periodontal health.
Secondly, the type of locally delivered antibiotic appeared to influence treatment outcomes. Different antibiotics, such as tetracycline, metronidazole, and minocycline, were commonly evaluated in the included studies. While the specific findings varied across studies, overall, locally delivered antibiotics demonstrated positive effects on periodontal parameters compared to mechanical debridement alone.
Moreover, the duration and frequency of local antibiotic administration were also found to impact treatment outcomes. Studies evaluating sustained-release delivery systems showed promising results, with prolonged antibiotic release leading to better periodontal improvement. Additionally, multiple applications of locally delivered antibiotics were associated with superior clinical outcomes compared to single applications.
Based on the review of the available evidence, it is our opinion that the use of locally delivered antibiotics as adjunctive therapy to nonsurgical periodontal treatment can provide valuable clinical benefits. These antibiotics can enhance the efficacy of mechanical debridement by targeting bacteria in challenging-to-reach areas and reducing bacterial load in periodontal pockets. However, it is important to consider the patient's clinical condition, risk factors, and potential side effects when determining the appropriateness of local antibiotic therapy.
CONCLUSION
Locally administered antibiotics offer several benefits in the treatment of periodontitis. They allow for targeted delivery, directly applying antibiotics to the affected sites, which enhances treatment efficacy. Compared to systemic administration, local delivery reduces the risk of systemic side effects, making it a safer option for patients. Additionally, localized treatment improves patient compliance by eliminating the need for multiple systemic doses. Furthermore, it helps minimize the development of bacterial drug resistance by limiting exposure to antibiotics. However, there are limitations to consider. Locally administered antibiotics may have limited reach and inconsistent penetration, which can compromise treatment effectiveness. Local adverse effects are also possible, requiring careful monitoring. Moreover, the evidence base and guidelines for their optimal use are still evolving. Understanding these benefits and limitations is crucial for making informed decisions regarding the use of locally administered antibiotics in periodontal therapy.
Strengths of the study include the comprehensive search strategy that utilized multiple databases, ensuring a thorough retrieval of relevant articles. The study also incorporated diverse sources by including papers on locally delivered antibiotics in periodontitis patients, antibiotics in periodontitis, and antimicrobial agents in periodontitis, enhancing the breadth of the included literature. The rigorous article selection process, conducted by two independent reviewers, increases the reliability and validity of the included studies. However, the study is subject to limitations, including potential language bias, as only English articles were considered, which may have excluded relevant studies published in other languages. Publication bias is another limitation, as only published articles were included, potentially introducing bias. Despite comprehensive searching, there is a possibility of missing relevant studies. The heterogeneity of the included studies in terms of design, sample size, interventions, and outcome measures may limit direct comparisons and synthesis. Additionally, the study lacked access to individual patient data, precluding meta-analysis and quantitative synthesis. Awareness of these strengths and limitations is crucial for interpreting the study's findings and implications.
Further research is warranted to assess the efficacy of local antibiotic administration in conjunction with nonsurgical periodontal therapy, utilizing larger sample sizes and longer follow-up periods. These studies will contribute to a better understanding of the benefits and limitations of locally delivered antibiotics in the treatment of periodontitis, thereby helping clinical decision-making in periodontal therapy. The findings of such research will help determine the optimal selection of antibiotics, dosage regimens, and delivery systems for various clinical scenarios. Additionally, investigating the long-term effects and potential complications associated with locally administered antibiotics will be crucial for ensuring patient safety. Furthermore, future studies should focus on developing standardized protocols and guidelines for the use of locally delivered antibiotics, which will aid in promoting consistency and best practices in periodontal therapy. Overall, continued research efforts in this field will provide valuable insights into the role of locally delivered antibiotics as adjunctive therapy, ultimately improving the outcomes and management of periodontitis.
LIST OF ABBREVIATIONS
| SRP | = Scaling and root planing |
| TE | = Tetracycline |
AUTHORS’ CONTRIBUTIONS
S.B. and M.A.T. contributed to the conception and design of the study. Both authors contributed equally to the analysis and interpretation of the references. Both authors contributed equally to writing the manuscript as well as to the drafted manuscript. M.A.T. revised it thoroughly. All authors read and agreed to the published version of the manuscript.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article is available in the the databases PubMed, Scopus and Google Scholar.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.


