All published articles of this journal are available on ScienceDirect.
Clinical and Radiological Benefits of Hyaluronic Acid in Periodontal Infrabony Defects: A Systematic Review and Meta-analysis
Abstract
Purpose:
There is no consensus on the clinical and radiological benefits of hyaluronic acid (HA) in patients with periodontitis having infrabony defects. Hence, this study examined the effects of HA in periodontitis patients with infrabony defects through a comprehensive systematic review process.
Methodology:
A systematic literature search was performed using PubMed/Medline, Scopus, Embase and Cochrane Library from inception to March 2022. Randomized or non-randomized clinical trials and single-arm clinical trials that assessed the clinical and radiological benefits of HA in periodontitis patients with infrabony defects with a minimum of 6 months follow-up were included in this study. Surgical regenerative therapy was considered as the comparator. The Cochrane risk of bias assessment tool and Downs and Black checklist was used for the quality assessment of randomized and non-randomized interventional studies, respectively. A subgroup and sensitivity analyses were performed to explore the heterogeneity and robustness of the findings, respectively.
Results:
A total of 13 out of 725 studies were included in this systematic review, of which 9 were considered for meta-analysis. The meta-analysis indicated significant benefits of HA in terms of reduction in probing pocket depth (SMD: 1.12 mm; 95% CI: 0.60-1.65; 9 studies), bone defect depth (SMD: 1.04mm; 95%CI: 0.62-1.47; 3 studies) and gain in clinical attachment level (1.04 mm; 95% CI: 0.33-2.47; 8 studies). Overall, the quality of included studies was good.
Conclusion:
The current evidence indicates that the administration of HA in the periodontal regenerative treatment of infrabony defects was significantly effective in increasing clinical attachment levels and reducing probing pocket and bone defect depth.
1. INTRODUCTION
Hyaluronic acid (HA) is one of the promising agents in the management of periodontitis in view of its biocompatibility, biodegradability, antimicrobial and wound healing properties. HA is a bio-molecule observed in various parts of the human body. It can be found in the gingiva, periodontal ligaments, cementum, alveolar bones, and in unstimulated saliva. It is a major constituent of the extracellular matrix and have a substantial role in cell migration and proliferation [1]. Ultimately, these features led to its periodontal effects like wound healing, tissue regeneration, and immunomodulation. HA is found to be effective in various medical therapies, its dental application is a growing research area in recent years [1].
The application of HA is observed to have beneficial effects in periodontitis with respect to many clinical and radiological outcomes like bleeding on probing, gain in periodontal attachment, probing pocket depth (PPD) reduction, gain in clinical attachment level (CAL), and reduction in bone height [1-3]. A review by Casale et al. [4] from 20 studies recorded beneficial effects in tissue repair, wound healing, and better quality of life along with the above said benefits. However, they recommended that well-designed randomized controlled clinical trials (RCTs) with adequate sample sizes are needed to confirm these promising results. Additionally, the available primary research provides conflicting conclusions regarding the effects of HA in periodontitis patients with infrabony defects [3, 5-7].
The systematic review conducted by Eliezer et al. [8] with 13 studies indicated a beneficial effect of HA with respect to the CAL gain and PPD reduction following the non-surgical and surgical management of periodontitis. However, high heterogeneity and risk of bias were the limitations of the studies. Another meta-analysis by Onisor et al. [9] from a limited number of studies recorded a significant increase in CAL gain and non-significant PPD reduction with adjuvant HA administration compared to the open-flap debridement.
To date, there are no meta-analyses known to the authors that assessed the radiological effects of HA in patients with infrabony defects following their surgical management. In view of these conflicts and limitations in the available evidence, we aimed to assess the clinical and radiological effects of the HA administered alone or in combination for the management of infrabony defects following the surgical intervention through a comprehensive systematic review and meta-analysis process.
2. MATERIALS AND METHODS
We followed a PICOS framework (Population, Intervention, Comparator, Outcome, and Study Design) for the inclusion of relevant studies and adapted the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Guidelines [10] to report this systematic review.
3. CRITERIA FOR CONSIDERING THE STUDIES FOR THIS REVIEW
3.1. Study Designs
Randomized or non-randomized clinical trials and single-arm clinical trials with pre-post analysis having a minimum of 6 months follow-up were considered in this review. Only the studies with full text available in the English language were considered. The observational studies, reviews, descriptive studies, commentary, guidelines, and qualitative analyses were excluded.
3.2. Participants
Adult patients with generalized periodontitis with infrabony defect who needed regenerative therapy irrespective of their gender were considered in this review. Studies involving the paediatric population were excluded.
3.3. Intervention
Application of HA or its derivative in different forms (like gel or mixed with other agents) or in different doses irrespective of the method of administration (alone or as an adjuvant therapy to surgical regenerative therapy) was considered. Studies that used HA along with non-surgical methods such as subgingival instrumentation or scaling and root planning were excluded.
3.4. Comparator
Surgical regenerative periodontal therapy without HA was considered as the comparator in the case of controlled trials. The pre-post analyses were considered in the case of single-arm studies.
3.5. Outcomes
Both clinical and radiological parameters were considered as outcomes which include i) bleeding on probing; ii) PPD reduction; iii) gain in CAL; iv) plaque accumulation/plaque index; v) Gain in bone probing depth from the cementoenamel junction (CEJ); vi) bone defect reduction/fill from CEJ; vii) changes in alveolar crest level; vii) reduction in marginal gingival level; viii) bone fill; ix) gingival recession; x) recession depth; and xi) bone crest depth.
3.6. Search Strategy
The databases such as PubMed/Medline, Scopus Embase, and Cochrane Library were accessed in March 2022 through a comprehensive search strategy. The strategy was prepared using all the possible keywords and entry terms for “Hyaluronic Acid” AND “Periodontitis”. We also did a snowball search in Google, Google Scholar, and ResearchGate to identify any relevant articles. Additionally, the Clinical Trial registry (https://clinicaltrials.gov/) and reference lists of relevant articles were also screened to identify additional relevant citations. The search strategy was prepared by the author with the support of the librarian. No date limit was used, and the search was limited to the English language. A detailed search strategy in various databases is provided in Supplementary file S1.
3.7. Study Selection
All articles identified following the database search were retrieved to an Excel sheet and screened against the pre-defined criteria. The studies were screened by reading its title and abstracts initially and followed by reviewing the full text of those included. Only those studies that passed both stages were considered for final inclusion in the review. Two independent reviewers were involved in the study selection, and any disagreements were resolved through discussion or consultation with another reviewer.
3.8. Data Extraction
The data were abstracted to a validated, comprehensive data extraction form. The author’s first name and year of publication were used to identify the studies. The data regarding the publication, study settings, participants, intervention, and outcomes were captured from the studies or calculated from the available data. The above data extraction form was finalized by trial and error by piloting on the first 2 articles.
3.9. Risk of Bias and Quality Assessment
The Cochrane Risk of Bias Assessment Tool was used to assess the methodological quality of included RCTs [11], and The Downs and Black checklist was used to assess the methodological quality of non-randomized clinical trials and single-arm studies [12]. The RCTs were graded as high, moderate, and low risk of bias based on the assessment. The single-arm studies were graded as excellent (26-28), good (20-25), fair (15-19) and poor (≤14) based on the score achieved.
3.10. Evidence Synthesis and Meta-Analysis
All the evidence extracted through the systematic process was summarized narratively and presented in tabular form. RevMan 5.4 was used to conduct the meta-analysis [13] wherever possible. The data were used as the difference in mean with standard deviation (SD), and outcomes were presented as standardised mean difference (SMD) along with a 95% confidence interval (CI). In studies that did not report an SD, the corresponding SD was calculated as per Cochrane guidelines [14]. If the same study reports the endpoints in different follow-ups, then the longest follow-up was considered for meta-analysis. Only studies that provided comparative data between the intervention and control group were considered for the meta-analysis, and the remaining evidence was presented narratively. The I2 statistics were used to estimate the heterogeneity in the analysis. We used the random effect model in case of substantial heterogeneity (I2>50%; P<0.10) and the fixed effect model in case of low heterogeneity (I2<50%; P>0.10). To explore the sources of heterogeneity, subgroup analysis was carried out based on the length of follow-up, wherever possible [15].
3.11. Publication Bias and sensitivity analysis
Assessment of evidence for publication bias through visual analysis of funnel plots or statistical methods was not performed, as less than 10 studies were used for the meta-analysis. Cochrane and previous literature recommend not performing funnel plot analysis in such cases [16-18]. The sensitivity analysis was performed to check the robustness of the findings by removing the study with the lowest weight in each analysis, and results were provided [18].
4. RESULTS
4.1. Study Selection Process
A total of 838 studies were identified from the databases and 4 studies from the additional searches. Out of these, 725 studies were screened using their title and abstract after removing the duplicate. Overall, 630 studies were excluded for various reasons, while 95 studies were eligible for full-text screening. A total of 82 studies were excluded during this stage; 13 studies were considered for systematic review, and 9 studies were included in the meta-analysis. The reason for excluding 4 studies from the meta-analysis is presented in Table 1. A detailed study selection process is depicted in Fig. (1).
4.2. Characteristics of the Included Studies
The studies were published between the years 2001 to 2021, with a majority of studies from India (n=4) [5-7, 19-22], followed by Egypt (n=3) [5, 21, 22], Italy (n=3) [3, 23, 24], and one each from Croatia [25], Brazil [26], and Sweden [2]. Among the included studies, 11 were RCTs, and the remaining two [24, 25] were single-arm studies. The duration of follow-up of the studies ranged from 6 months to 24 months. A detailed study and subject characteristics are presented in Table 2.
| S.No. | Study ID and Reference Number | Used in Systematic Review | Reason for Excluding from Quantitative Estimation |
|---|---|---|---|
| 1 | Božić D et al., 2021 [25] | Yes | Single arm clinical study provided the effects of HA before and after treatment among the included patients. |
| 2 | Pilloni A et al., 2021 [23] | Yes | Study another regenerative material which is having well documented effects and the study did not include a negative control. |
| 3 | Mohamed MO et al., 2020 [21] | Yes | The study reported outcomes in percentage changes which is heterogeneous from other studies. |
| 4 | Vanden Bogaerde L. et al., 2017 [24] | Yes | Single arm clinical study provided the effects of HA before and after treatment among the included patients. |
| Study ID/Refs. | Reg No. | Country (State/Sites) | Study Design | Study Duration | Number of Participants and Implants in the Final Analysis | Duration of Follow up |
Age (years) Mean (SD) |
Gender (Male/Female; %) |
|---|---|---|---|---|---|---|---|---|
| Bhowmik E et al., 2021 [7] | CTRI/2017/11/010591 | India | Prospective, randomized, split-mouth clinical study | NR | 8 patients with 32 graftable sites (18 test and 14 control) | 12 months | 36.9 (7.49) | 25/75 |
| Sehdev B et al., 2016 [19] | NR | India | Parallel group RCT | NR | 20 patients with 24 interproximal defects (12 test and 12 control) | 6 months | 32.16 (8.12) | NR |
| Božić D et al., 2021 [25] | NR | Croatia | Single arm clinical study | June 2019 and December 2020 | 23 patients | 6 months | 54.59 (10.24) | 30.4/69.6 |
| El-Sayed KMF et al., 2012 [5] | NR | Egypt | Prospective, randomized, split-mouth clinical study | NR | 14 patients (14 test and 14 control sites) | 6 months | NR | NR |
| Mamajiwala AS et al., 2021 [6] | CTRI/2018/03/012334 | India | single-centre, double-blind, split-mouth RCT | April 2017 to November 2018 | 20 patients (20 test and 20 control sites) | 12 months | 39.9 ± 4.18 | 55/45 |
| Pilloni A et al., 2021 [23] | NR | Italy | RCT | September 2016 to October 2019 | Intervention: 16 subject (16 intrabony defects) | 24 months | 41.19 ± 8.49 | 50/50 |
| Control: 16 subject (16 intrabony defects) | 41.75±10.22 | 56/44 | ||||||
| de Santana RB et al., 2015 [26] | NR | Brazil | Prospective split-mouth RCT | NR | 30 patients (30 test and 30 control defects) | 12 months | 49.2 | 36/64 |
| Selvaprakash K et al., 2021 [20] | NR | India | Prospective RCT | January 2017 to December 2017 |
16 patients with 30 sites (15 test and 15 control sites) | 6 months | Test: 48.5; Control: 45.7 | 44/56 |
| Engström et al., 2001 [2] | NR | Sweden | Prospective split-mouth RCT | NR | 6 individuals | 12 months | 49 | 66/34 |
| El-Wakeel NM et al., 2017 [22] | NR | Egypt | Blinded RCT | NR | Intervention: 15 patients | 12 months | 33.6±7.23 | 55/45 |
| Control 1: 15 patients | 34.17±6.5 | |||||||
| Control 2: 15 patients | 31.8±6.02 | |||||||
| Mohamed MO et al., 2020 [21] | NR | Egypt | RCT | NR | 16 patients with 20 defects (5 each to Intervention 1 & 2; control 1&2) | 6 months | NR | NR |
| Vanden Bogaerde L. et al., 2017 [24] | NR | Italy | Single arm study | NR | 16 patients with 19 infrabony defects |
12 months | Range: 35-67 | 25/75 |
| Briguglio F et al., 2013 [3] | NR | Italy | RCT | NR | Intervention: 20 | 24 months | 47.7±8.1 | 8/12 |
| Control: 20 | 42.3±8.4 | 10/10 |
4.3. Characteristics of the Treatment and Outcomes
Strict inclusion and exclusion criteria were used by all the studies to include their participants. All studies included adult participants with periodontitis. HA was administered alone or in combination with other agents such as bioresorbable membrane [19], deproteinized porcine bone mineral xenograft [25], recombinant human Fibroblast Growth Factor type 2 [26], β-tricalcium phosphate [20], and bone allograft [22] following surgical procedure as an intervention. Various clinical and radiological outcomes were considered by the studies, as presented in Table 3.
4.4. Quality Assessment of Included Studies
Among the included RCTs, 45% (n=5) of the studies were observed to have a high risk of bias and low risk of bias (n=5, 45%), whereas only a single study [21] had a moderate risk of bias in terms of randomization. Allocation was concealed in 54% (n=6) of the studies, which is observed to have a low risk of bias in this domain, and the remaining studies did not report the allocation. Six (54%) of the studies were blinded (the study participants did not know the treatment they received), whereas the remaining five (46%) studies didn’t report it. The risk of bias on outcome assessor blinding was high, moderate, and low in three (27%), five (46%) and three (27%) of the studies, respectively. The risk of bias with respect to the incomplete outcome data, selective reporting and other biases was observed to be low. The two single-arm interventional studies [24, 25] were observed to have a fair quality, as both of them scored 17 out of 28. The quality assessment analysis is provided in Supplementary file S2.
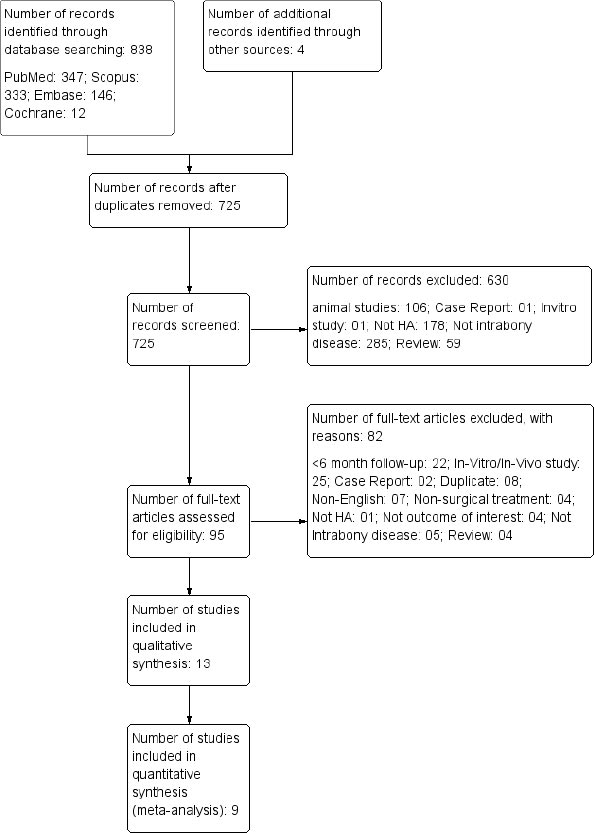
Table 3.
| Study ID/Refs. | Inclusion criteria/Diagnosis | Exclusion Criteria | Intervention/Comparator/ Duration of Treatment | Outcomes/Endpoint | Outcomes Observed | Author’s Key Conclusion |
|---|---|---|---|---|---|---|
| Bhowmik E et al., [7] | Generalized chronic periodontitis patients ; 30-55 years of age, and having contralateral infrabony pockets measuring ≥6 mm; radiographic evidence of bilateral vertical/angular defects; and consenting patients who were ready for regular follow-up | Patients with a history of periodontal or antibiotic therapy in the past 6 months, pregnant/lactating women, smokers, medically compromised, or under therapeutic regimen that may alter the probability of soft tissue and bone healing |
Intervention: Hyalouranan-nano hydroxyapatite bone graft after a flap surgery Control: nano hydroxyapatite bone graft after flap surgery |
PPD; CAL; bone probing depth; amount and percentage of defect depth reduction and change in alveolar crest level | Statistically significant improvements in all the parameters were seen in the test sites when compared to control sites at 12 months. | HA-nano hydroxyapatite bone graft can be considered a promising periodontal regenerative material |
| Sehdev B et al., [19] | Systemically healthy patients; presence of at least one radiographically detectable interproximal infrabony osseous defect with probing pocket depth (PPD) ≥5 mm and clinical attachment loss ≥5 mm following initial therapy; depth of intra-osseous component of the defect ≥3 mm by clinical and radiographic means and later confirmed intrasurgically; a radiographic base of the defect at least 3 mm coronal to the apex of the test teeth and the presence of at least 3 mm width of keratinized gingiva around test teeth | Patients with unacceptable oral hygiene (plaque index >1); smokers or who used any of tobacco products; study tooth with inadequate endodontic/restorative treatments; clinical or radiographic signs of untreated acute infection, apical pathology, root fracture, severe root irregularities, cemental tears, cementoenamel projections not easily removed by odontoplasty, untreated carious lesions at CEJ or on the root surface at the selected site; study teeth showing mobility exceeding grade II, and class III/class IV furcation defect; pregnant females or lactating mothers and evidence of localized aggressive periodontitis. |
Intervention: HA in combination with bioresorbable membrane after flap surgery Control Bioresorbable membrane alone after flap surgery |
PPD, CAL, and relative gingival margin level CEJ to base of bone defect, CEJ to root apex and radiographic defect depth. Linear bone growth and percentage of bone fill |
Treatment was effective in reduction of PPD and radiographic defect depth with a higher CAL gain | HA treatment was effective in clinical and radiographic benefits |
| Božić D et al., [25] | Diagnosis of stage III or IV periodontitis; good general health with no systemic diseases that could contraindicate surgery, no medications that could affect the periodontal status, uncontrolled or poorly controlled diabetes, no pregnancy or lactation; | Teeth with degree III mobility, furcation involvement, or inadequate endodontic treatment and/or restoration; heavy smokers (more than 10 cig/day). |
Intervention: Mixture of cross linked HA and deproteinized porcine bone mineral xenograft after regenerative periodontal surgery Control: No control group (Pre-post analysis) |
PPD; CAL and GR | Applying a combined HA and xenograft approach in deep infrabony defects provides clinically relevant CAL gains and PPD reductions compared to baseline values and is a valid new approach in treating infrabony defects. | HA was significantly effective |
| patients had to have at least one infrabony defect with PPD ≥ 6 mm, CAL ≥ 6 mm and an infrabony component ≥ 4 mm measured on digital periapical radiographs that predominantly involved the interproximal area of the affected tooth; FMPS and FMBS ≤ 20% following non-surgical treatment; vital teeth or teeth with properly performed endodontic treatment | - | - | - | - | - | |
| El-Sayed KMF et al., [5] | Chronic periodontitis cases with at least four interproximal sites with moderate to deep infrabony defects (≥3 mm) on the radiographs, and clinical probing depths (PD) >5 mm on premolars or molars following initial nonsurgical periodontal therapy, with a minimum of 20 teeth in each patient. | Received antibiotic therapy or periodontal treatment within the 6-month period prior to the study |
Intervention: WMF with adjunctive 0.8% HA gel application Control: WMF with placebo gel |
CAL, PPD, GR PI, and BOP | Statistically significant differences were noted for CAL and GR in favour of the test sites; No significant differences were found regarding PD, BOP, or PI values; | HA gel application appears to improve the clinical outcome of MWF surgery |
| Mamajiwal AS et al., [6] | Patients diagnosed with chronic periodontitis having at least two contralateral infrabony periodontal defects with probing pocket depth ≥5mm and infrabony defect ≥ 3mm; Individuals were currently classified with periodontitis stage II or III (grade A to B) as per new guideline; Periodontal defects were divided into two-wall infrabony defect and three-wall infrabony defect in the age group of 30–58 years. | Patients diagnosed with aggressive periodontitis, having interproximal craters, one-walled infrabony defect, grade III furcation involvement, presence of caries or overhanging restorations, presence of peri-apical injuries, pregnant and lactating women, smokers, patients with any known allergies, and patients failing to observe the recommended oral hygiene measures (full mouth PI scores > 1.5). |
Intervention: 0.8% hyaluronic acid and open flap debridement Control: Open flap debridement |
CAL, PPD, GR , base of infrabony defect, alveolar crest, depth of the infrabony component of the defect, defect fill, alveolar crest changes, and defect resolution | Test group showed significantly greater CAL gain and bone defect fill compared to the control group. Mean PD reduction in the test group was statistically significant compared to the control group at 12-month period. | Application of HA acid gel in conjunction with open flap debridement resulted in enhanced clinical and radiographic outcomes compared to open flap debridement alone. |
| Although only interproximal infrabony defects in relation to multirooted teeth were selected, teeth with grade I furcation and grade II furcation were inadvertently included in some cases. Systemically healthy subjects not receiving antibiotic therapy or periodontal treatment during a 6-month period prior to the study | - | - | - | - | ||
| Pilloni A et al., [23] | Adults aged 18–65 years with periodontal disease; good physical health; sites with infrabony defects on single-rooted teeth and persisting pockets (probing depth (PD) ≥ 6 mm and bleeding on probing (BOP)) at re-evaluation 6 weeks after non-surgical periodontal therapy; radiographic infrabony component ≥ 3 mm; limited to no extension of the defect on the lingual or palatal side as assessed by preoperative bone sounding and FMPSFMBS ≤ 20% before surgery | Relevant medical conditions contraindicating surgical interventions; pregnancy or lactation; tobacco smoking; untreated periodontal conditions; any condition associated with poor compliance or failure to maintain good oral hygiene; acute infectious lesions in areas intended for surgery; teeth with grade 2 or higher mobility; and restorations or carious lesions on root surfaces that are associated with the infrabony defect. |
Intervention: Application of cross-linked HA gel following regenerative periodontal surgery using the single-flap approach Control: Enamel matrix derivative following regenerative periodontal surgery using the single-flap approach |
CAL, PPD, GR, and BOP | At 24 months, both treatments resulted in statistically significant clinical improvements evidenced by PD-reduction and CAL-gain. PD-reduction was statistically significantly higher for the control group than the test group. Test sites showed slightly lower GR values than the control sites | Application of EMD resulted in statistically significantly higher PD-reduction compared to the use of HA. |
| de Santana RB et al., [26] | Adult subjects with chronic periodontitis presenting; radiographic evidence alveolar bone loss at the proximal aspect of the tooth; infrabony defect deeper than 4 mm probing pocket depth >6 mm at the site; unremarkable general health according to medical history and clinical judgment; no medications taken for at least six months and no antibiotics taken for twelve months before the beginning of the study; and non-smokers |
Subjects with “early onset” or aggressive forms of periodontitis; current smokers; presence of significant systemic diseases (i.e., cancer, AIDS, diabetes; clinical evidence of furcation defects; presence of apical radiolucency and previous lack of cooperation with the maintenance program. |
Intervention: Open instrumentation of the root surfaces and bone defects via flap modified papilla preservation flap and application of a recombinant human Fibroblast Growth Factor type 2 in a hyaluronic acid carrier Control: Open instrumentation of the root surfaces and bone defects via flap modified papilla preservation flap |
PPD, GR, probing attachment level and probing bone level | Test sites exhibited significantly more PD reduction, PAL gains and shallower residual PD than controls. Moreover, residual PD smaller than 5 mm and PAL gain > 4 mm was significantly more frequent in the test group | Application of HA significantly improved clinical parameters of periodontal wound healing |
| Selvaprakash K et al., [20] | Isolated sites with Probing Pocket depth ≥5 mm (following root surface debridement) having a two or three walled infrabony defect in the posterior teeth | Smokers, pregnant women, patients with systemic disease/condition influencing course of periodontal disease (diabetic patients, chronic systemic diseases such as rheumatoid arthritis, renal, hepatic and pulmonary diseases; allergies and patients who underwent periodontal treatment in past six months and those taking medications (corticosteroids or bisphosphonates) influencing bone metabolism |
Intervention: 0.8% HA and β-TCP with followed by Root surface/defect debridement (regenerative osseous surgery) Control: β-TCP followed by Root surface/defect debridement (regenerative osseous surgery) |
PPD, CAL and bone defect level | The PPD was significantly reduced, CAL and bone fill were increased in both groups from baseline, indicating clinical effectiveness. From baseline to six months, the PPD reduction and gain in CAL in the test and control groups were was statistically significant. | HA as an adjunct to β-TCP demonstrated only comparable outcomes |
| Engström et al., [2] | Diagnosis of chronic periodontitis; The selected teeth displayed defects of similar character and magnitude, and there were at least 2 teeth between the test and the control tooth both in the same jaw; patients with infrabony pockets and pockets >6 mm deep | Having received antibiotics 1 month prior to treatment or metronidazole (topical or oral) 6 months prior to treatment; receiving treatment for allergy; hypersensitivity to amalgam or other metals; previous radiation treatment (head or neck); alcohol or drug abuse; or other serious disease |
Intervention: Bioabsorbable membrane with HA (0.85 ml injection) was placed in the infrabony pocket following debridement and root preparation Control: Bioabsorbable membrane following debridement and root preparation |
Gingival crevicular fluid immunoglobulin (Ig)G, C3, and prostaglandin E2 (PGE2) responses; PPD; BOP; and the presence of plaque | For the surgical treatments, bone height was increased in the test group treated with HA and reduced in the control group which was significant (P<0.05) after 12 months. | HA was significantly effective in increasing bone height among the patients undergone surgical management |
| El-Wakeel NM et al., [22] | Systemically healthy, no contraindications to surgery, no abnormal platelet count, had at least one vertical osseous defects with probing pocket depth of >5 mm and clinical attachment loss <5 mm along with radiographic evidence of vertical/ angular bone loss in the affected sites and had at least 2 mm of keratinized gingiva on facial aspect of selected tooth |
Smokers, ex-smokers, patients allergic or sensitive to any medication and/or local anaesthesia, patients showing unacceptable oral hygiene compliance, pregnant and lactating females and patients with teeth with excessive mobility |
Intervention: Bone graft + hyaluronic acid following the surgical procedure using a conventional periodontal flap surgery Control 1: bone graft+ Platelet-poor plasma following the surgical procedure using a conventional periodontal flap surgery Control 2: Bone graft alone following the surgical procedure using a conventional periodontal flap surgery |
PPD, CAL and radiographic bone fill | A statistically significant improvement in CAL and PPD as well as radiographic bone fill were reported in HA group and platelet compared to bone graft alone | HA with bone graft was effective than the bone graft alone in patients with periodontitis |
| Mohamed MO et al., [21] | Free from any systemic disease, having moderate, advanced chronic periodontitis with at least one site with clinical attachment loss ≥ 5mm, non-smoker and non-pregnant women | NR |
Intervention 1: Mucoperiosteal flap along with 0.8 percent HA gel Intervention 2: Mucoperiosteal flap with combination of PRF and 0.8% HA gel Control 1: Mucoperiosteal flap with application of (PRF) alone Control 2: Mucoperiosteal flap |
PPD, CAL, PI and GI Gingival index | Clinical improvement was noticed statistically in each group through different intervals. nevertheless, non-significant difference had been reported upon comparing different groups regarding percent change in GI, PPD and CAL | The local application of HA gel alone improve the clinical outcomes more than open flap debridement |
| Vanden Bogaerde L. et al., [24] | Periodontal patients with a defects of probing depth of at least 6 mm | Patients with unstable medical conditions, poor oral hygiene, or a smoking habit |
Intervention: Esterified hyaluronic acid in the form of fibres (Hyaloss matrix, Meta) following the surgery of flap raising Control: No control; Pre-post analysis |
PPD, GR , CAL | The mean PPD was reduced, gingival recession and CAL gain had increased, after one year treatment | One year treatment with HA was clinically effective in patients with infrabony disease |
| Briguglio F et al., [3] | Absence of systemic disease, negative history for pregnancy, no regular use of medication or drugs for last 6 months, non-smoking, advanced generalized chronic periodontitis, plaque index of <1, presence of an angular two wall infrabony defect in the interproximal area (PD≥7mm; CAL≥7mm), absence of furcation involvement or angular defects extending into the furcation area of the target tooth, absence of carries or overflowing restoration, and absence of periapical injuries | NR |
Intervention: Esterified hyaluronic acid in the form of fibres (Hyaloss matrix, Meta GCM) following the surgery using simplified papilla preservation flaps Control Surgery using simplified papilla preservation flaps |
PPD, distance between CEJ to residual ridge bone and base of the defect; plaque index and CAL | A significantly better CAL gain and PPD reduction was observed in the test than the control. | The treatment of with HA offered an additional benefit compared to treatment with open flap debridement. |
5. CLINICAL EFFECTS OF HA
5.1. Probing Pocket Depth
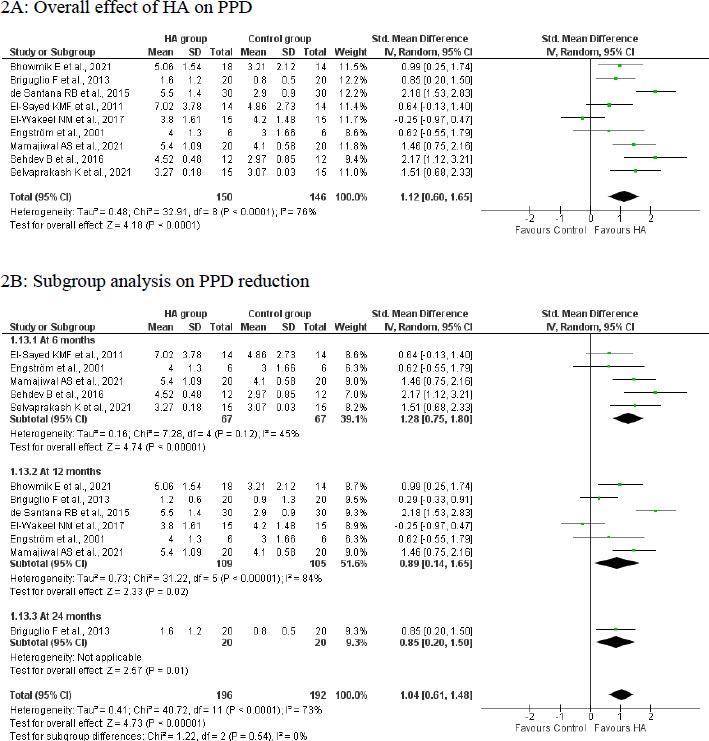
A meta-analysis of 9 studies with 296 participants indicated that treatment with HA significantly reduced PPD by 1.12 mm (95%CI: 0.60 to 1.65; p<0.0001; (Fig. 2A) compared to the control group in patients with infrabony defects. However, there was a significant level of heterogeneity (I2: 84%; 76%); hence random effect model was applied.
5.2. Subgroup Analysis
A subgroup analysis based on the length of follow-up indicated that treatment with HA was able to achieve a significantly greater benefit in terms of PPD reduction at 6 months (SMD: 1.28; 95%CI: 0.75 to 1.80; p<0.00001; n=5 studies; 134 participants; I2: 45%; Fig. 2B), 12 months (SMD: 0.89; 95%CI: 0.14 to 1.65; p=0.02; n=6 studies; 214 participants; I2: 84%; Fig. 2B) and 24 months (SMD: 0.85; 95%CI: 0.20 to 1.50; p=0.01; n=1 study; 40 participants; Fig. 2B) compared to the control group. A reduction in heterogeneity was observed in the analysis on the 6-month follow-up but not in the 12-month follow-up.
A single-arm study by Božić D et al. [25] recorded a significant reduction (p<0.001) of 4.54 ± 1.65 mm in PPD following 6-month treatment in 23 patients with 27 infrabony defects who were treated with a combination of HA and deproteinized porcine bone mineral following regenerative periodontal surgery. Another study by Vanden Bogaerde L et al. [24] among 19 defects indicated a mean reduction of 5.8 mm in PPD following one-year treatment with HA in patients with infrabony defects. None of the studies recorded PPD reduction at 9 months. The study conducted by Pilloni A et al. [23] recorded a significantly (p= 0.001) better PPD reduction in the enamel matrix derivatives group (4.5±0.97 mm versus 3.31±0.70 mm) compared to the HA group. In contrast, a non-significant effect was reported by Mohamed et al. [21] with hyaluronan gel.
5.3. Gain in CLinical Attachment Level
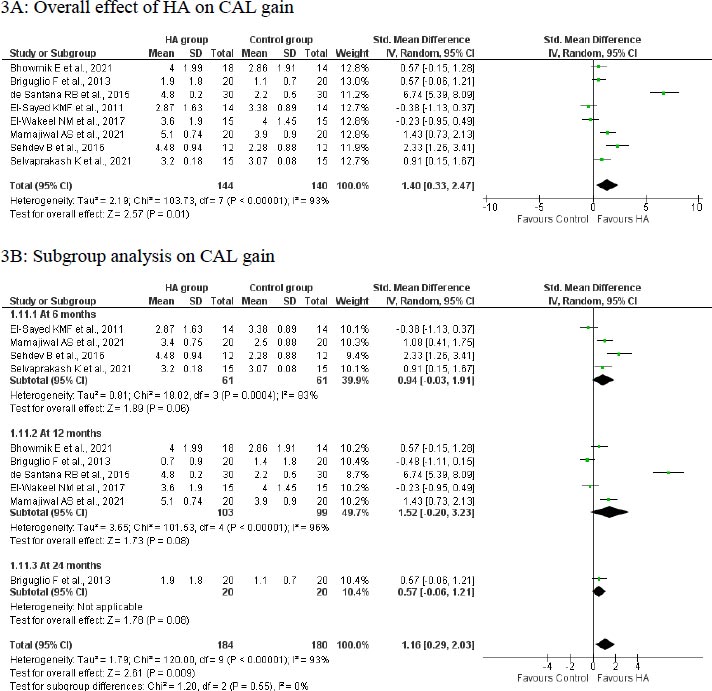
A meta-analysis of 8 studies with 284 participants indicated that treatment with HA was significantly effective in a CAL gain of 1.40 mm (95%CI: 0.33 to 2.47; p<0.00001; Fig. 3A) compared to the control group in patients with infrabony defects. However, there was a significant level of heterogeneity (I2: 84%; 93%); hence random effect model was applied.
5.4. Subgroup Analysis
The meta-analysis of studies indicated a beneficial effect of HA on the CAL gain at 6 months (SMD: 0.94; 95%CI: -0.03 to 1.91; 4 studies; 122 participants; I2: 83%; Fig. 3B), 12 months (SMD: 1.52; 95%CI: -0.20 to 3.23; 5 studies; 202 participants; I2: 96%; Fig. 3B) and 24 months (SMD: 0.57; 95%CI: -0.06 to 1.21; 1 study; 40 participants; Fig. 3B) in patients with infrabony defects. However, this effect in subgroups were not statistically significant (p>0.05). The heterogeneity was not altered following the subgroup analysis.
5.5. Plaque Accumulation Index
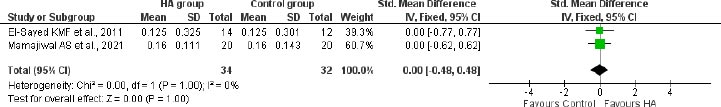
A meta-analysis of 2 studies with 66 participants recorded no significant difference between the HA-treated group and control group in terms of plaque accumulation index at 6 months (SMD: 0.00; 95%CI: -0.48 to 0.48; 2 studies; 66 participants; I2: 83%; Fig. 4) in patients with infrabony defects.
5.6. Bleeding on Probing
Pilloni et al. [23] assessed the effect of HA versus enamel matrix derivative following the single-flap in patients with infrabony defects. They recorded a non-significant change in BOP at 12, 18, and 24 months in the groups. Similarly, the study conducted by El-Sayed et al. [5] recorded a non-significant benefit following the HA application in conjunction with periodontal surgery. The number of bleeding sites decreased over the treatment in a study reported by Engström et al. [2], whereas it increased as per the findings by Briguglio et al. [3].
6. RADIOLOGICAL EFFECTS OF HA
6.1. Reduction in Bone Probing Depth
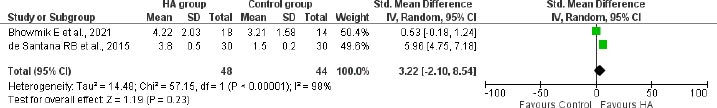
The meta-analysis of 2 studies with 92 participants revealed an additional reduction of 3.22mm (95%CI: -2.10 to 8.54) bone probing depth from CEJ at 12 months in the the HA group compared to the control group in patients with infrabony defects (Fig. 5).
6.2. Reduction in Bone Defect Depth
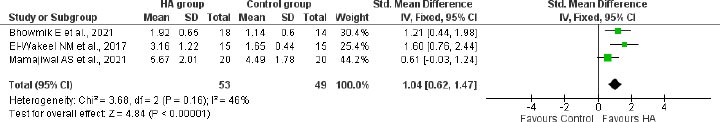
A meta-analysis of 3 studies with 102 participants revealed a significantly greater reduction of 1.04 mm (95%CI: 0.62 to 1.47) in bone defect depth from CEJ at 12 months in patients treated with HA compared to the control group in patients with infrabony defects (Fig. 6).
6.3. Changes in Alveolar Crest Level
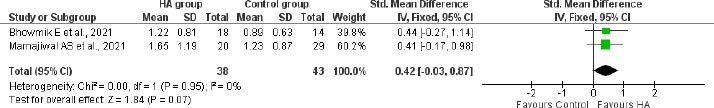
A meta-analysis of 2 studies among 81 participants indicated a change of 0.42mm (95% CI: -0.03 to 0.87) in alveolar crest level in HA treated group compared to the control group following 12 months of treatment in patients with infrabony defects (Fig. 7).
6.4. Gingival Recession
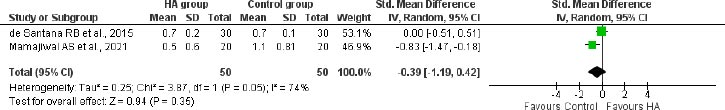
A meta-analysis of 2 studies with 100 participants indicated no significant difference in gingival recession (SMD: -0.39 (95%CI: -1.19 to 0.42)) between the HA-treated group and control group following 12 months of treatment in patients with infrabony defects (Fig. 8).
6.5. Sensitivity Analysis
The sensitivity analyses were performed by removing the study with the lowest weight. The study by Engström et al. [2], with a weight of 8.6%, and de Santana RB et al. [26], with a weight of 11.2%, were removed in PPD reduction and CAL gain analysis, respectively. The results were robust, as there was no alteration in the overall effect. The result of sensitivity analyses is provided in Supplementary file S3
7. DISCUSSION
HA is a natural polysaccharide of the extracellular matrix of connective tissue, synovial fluid, and other tissues. HA exhibits many biological functions, such as cellular and extracellular interactions, growth factors interaction, osmotic pressure homeostasis, and tissue lubrication. These properties helped HA to achieve wide therapeutic applications in various fields of cosmetics, medicine and pharmaceuticals [27].
HA has a wide variety of applications in many medical conditions, such as osteoarthritis as a dermal filler [28], ophthalmic, dermal, burns, wound repair, skin aging and other health conditions [29, 30]. Recently, HA has become an attractive topic in the dental industry and has become a boon in periodontal therapy due to its anti-inflammatory and anti-bacterial effects [27].
This systematic review assessed the clinical and radiological effects of HA in periodontitis patients with infrabony defects. We included a total of 13 studies published from 2001 to 2021 from various parts of the world. Majorly, of the studies were from India, Egypt and Italy. The HA was used with various other treatment options following surgical management. The included RCTs were observed to have a low risk of bias, and single-arm interventional studies included in the review had fair quality.
Our meta-analysis indicated a significant PPD reduction of 1.12 mm in the HA-treated group compared to the control group. A similar significant reduction of PPD was observed in the subgroup analysis at 6 months (1.28 mm), 12 months (0.89 mm) and 24 months (0.85 mm) of therapy with HA compared to the control group in patients with infrabony defects. This was in line with the findings reported by Onsior et al. [9]. In which, they have reported a 1.11 mm PPD reduction in HA treated group compared to the open flap detriment. All the single-arm studies also recorded a significant PPD reduction following the HA treatment with surgical procedure, though not with reports by Mohammed et al. [21]. Similarly, another previous meta-analysis by Eliezer et al. [8] recorded a 0.89 mm PPD reduction after 6-24 months of treatment with HA compared to surgery.
There was a significantly greater CAL gain (1.40 mm) in the HA-treated group compared to the control group who received the surgical procedure in patients with infrabony defects. A similar CAL gain was observed in the subgroup analysis at 6 months (0.94mm), 12 months (1.52mm) and 24 months (0.57mm) of the management. Similar findings were recorded by the meta-analysis conducted by Onsior et al. [9] and Eliezer et al. [8], where they recorded a 1.38 mm and 0.85 mm CAL gain in the HA group compared to surgery, respectively.
The previous meta-analyses [8, 9] did not quantify other efficacy parameters of HA, and here we tried to assess the clinical and radiological efficacy parameters of HA with the available data. Our meta-analysis indicated that the treatment with HA was significantly effective in reducing the bone defect depth in 12 months (Fig. 6) in patients with infrabony defects. Only 3 studies [6, 7, 22] recorded this outcome, and further clinical studies should analyze the effect of interventions on reducing bone defect depth as it is an important parameter to decide the efficacy of management. However, the treatment with HA was not effective in improving the plaque index or bone probing depth (Fig. 5), and alveolar crest level (Fig. 7) over surgical intervention. It should be noticed that all studies included patients with good oral hygiene and low plaque index in starting with, which could explain the lack of significant changes in plaque index after the intervention.
This study has several strengths. i) All the 9 included RCTs in the meta-analysis were observed to have a good methodological quality with a low risk of bias. Hence, the interpretation drawn from this meta-analysis can be adopted in clinical practice. ii) In this study, we tried to capture the maximum possible clinical and radiological benefits of HA in patients with infrabony defects. iii) Our sensitivity analysis by removing the studies with the lowest weight revealed the robustness of our findings by yielding a non-differing result from the original results. iv) A subgroup analysis with respect to the length of follow-up could help us to understand the effect of HA with respect to its treatment duration; however, future studies should report detailed subgroup data to strengthen these findings.
The restriction to the English language studies was a limitation in our meta-analysis. However, a comprehensive search in numerous databases might have helped us to collate the maximum available resources. There was a high level of heterogeneity in the analyses; hence caution should be taken while interpreting the results. Moreover, a subgroup analysis based on the length of follow-up showed variation in the heterogeneity; hence future studies should focus on other factors that can influence the outcomes. Additionally, only a few studies recorded the radiological benefits of the interventions, and future studies should also focus on these outcomes, as this is also an important parameter regarding the effectiveness of the treatment's success. Further studies are needed across different populations of the world to better characterize the treatment outcomes in all different settings with more focus on the radiological outcomes of HA.
CONCLUSION
The current evidence indicates that the administration of HA in the periodontal regenerative treatment of infrabony defects was significantly effective in increasing clinical attachment levels and reducing probing pocket and bone defect depth.
LIST OF ABBREVIATIONS
| HA | = Hyaluronic acid |
| PPD | = Probing pocket depth |
| CAL | = Clinical attachment level |
| RCTs | = Randomized controlled clinical trials |
| CEJ | = Cementoenamel junction |
| SD | = Standard deviation |
| SMD | = Standardised mean difference |
| CI | = Confidence interval |
CONSENT FOR PUBLICATION
Not applicable.
STANDARDS OF REPORTING
PRISMA guidelines are followed.
FUNDING
No financial support was received for this manuscript.
CONFLICT OF INTEREST
The author reports no conflicts of interest in this work.
ACKNOWLEDGEMENTS
We thank Prof Hani Almoallim for his expertise, insights, and assistance in the development of the systematic review.
SUPPLEMENTARY MATERIAL
PRISMA checklist is available as supplementary material on the publisher’s website along with the published article.
Supplementary material is available on the publisher’s website along with the published article.


