All published articles of this journal are available on ScienceDirect.
Cytotoxic Activity of Gambier Leave (Uncaria gambir) Ethyl Acetate Extract on Mouse Embryonic Fibroblast Cell (NIH-3T3) using MTT Assay
Abstract
Background:
Uncaria gambir or gambier is one of the plants widely found in Indonesia. Gambier is locally known as an antioxidant and antibacterial agent because it has high catechin content. Ethyl acetate extract of gambier leaves has been investigated to contain the highest catechin content than other extraction solvents. Fibroblasts are often used in biomaterial viability and toxicity tests because they have a highly reproducible growth rate and biological response. NIH-3T3 is commonly used as a substitute for human gingival fibroblasts. However, no study has been conducted on the cytotoxic activity of gambier extract on fibroblast cells.
Objective:
The aim of this study is to evaluate whether the cytotoxic activity of gambier ethyl acetate extract (GEE) exerts on NIH-3T3 cell lines using MTT assay.
Methods:
The cytotoxic activity of gambier extract was evaluated in three incubation periods. The cytotoxicity test was conducted using an ethyl acetate extract of gambier (Uncaria gambir Roxb.) leaves. The NIH-3T3 cell was treated by GEE in ten concentrations (0, 2.5, 5, 10, 25, 50, 100, 250, 500, and 1000 ppm) for 24-, 48, and 72-hour incubation periods. Cell viability was determined with MTT (3-4,5-dimethylthiazol-2yl -2,5-diphenyltetrazolium bromide) assay. The data were analyzed statistically using SPSS based on ANOVA, followed by Tukey HSD post hoc with p<0.05 and ANOVA paired sample T-test with p<0.05, and the CD50 value was measured by Sigma Plot software.
Results:
GEE at 2.5, 5, 10, 25, 50, 100, and 250 ppm have cell viability >80%, and cell viability was observed to be increased based on the incubation period. GEE at 1000 ppm significantly decreased the cell viability from GEE at 0 ppm in 24-, 48-, and 72-hour incubation periods (23.83%, 30.14%, and 19.02%, respectively). Moreover, GEE at 500 ppm became toxic by significantly decreasing the cell viability in 48- and 72-hour incubation (40.43% and 23.03%, respectively). The CD50 value of GEE at 24-, 48-, and 72-hour incubation was found to be 578.03 ppm, 488.63 ppm, and 470.70 ppm, respectively.
Conclusion:
GEE at 2.5, 5, 10, 25, 50, 100, and 250 ppm were not found to be toxic to NIH-3T3 cells for 24-, 48-, and 72-hour incubation periods.
1. INTRODUCTION
Plants have been used as one of the most important sources of medicine. Indonesia, with its wide biodiversity, has plenty of resourceful natural materials that should be explored. Gambier (Uncaria gambir Roxb) is a plant that widely grows in Indonesia, such as Riau, West Sumatra, South Sumatra, Bangka Belitung, and East Kalimantan [1]. Gambier extract is widely used as an herb to cure diarrhea, canker sores, dysentery, mouth sores, and skin pain [2]. The previous study investigated that U. gambir comprises 7.63-23.16% water, 12.24-24.16% tannins, 14.76-86.71% catechins, 1.43-25.24% ash, as well as 5.58-46.28% water-insoluble compounds [3-5]. Based on a previous study, ethyl acetate extract from gambier leaves has been investigated. It was found that catechin content is higher than other solvents, such as methanol and hot water [6]. Catechin is a secondary metabolite characterized as a flavonoid. Catechin is the main compound in various plants, such as green tea, cocoa, beans, and gambier extract [7]. Studies on catechin in vitro and in vivo have revealed that it has antioxidant, anticarcinogenic, anti-inflammatory, antiatheroge-nic, and hypoglycemic properties, as well as antibacterial and antiviral properties [8]. However, further studies on ethyl acetate extract need to be conducted.
Gambier extract has the potential to act as antibacterial, antioxidant, and anti-fungal in oral treatment [9]. A previous study evaluated gambier extract as an antioxidant using DPPH methods. The result showed that the DPPH inhibitory activity of ethanol and ethyl acetate extracts was higher than that of aqueous extracts. Moreover, the IC50 values of ethanol and ethyl acetate extracts were in the range of 13.8 to 16.2 μg/mL, and the IC50 values of aqueous extracts were 27.4 μg/mL [10]. The antioxidant activity of gambier extract was also evaluated by lipid peroxidation. Gambier extract showed anti-lipid peroxidation against Fe2+- induced lipid peroxidation at 250 μM concentration [11]. In a study conducted by Nordin et al., gambier extract exhibited higher inhibitory effects in lipid peroxidation than α-tocopherol [12]. In general, gambier extracts have high antioxidant activities [13] and have been evaluated as antibacterial agents. In a study, gambier demonstrated antibacterial activity by decreasing the value of the Total Bacterial Plate Number (TPN) for the additive of hand soap [14]. Moreover, gambier extract also inhibited the Streptococcus mutans [15]. A previous study showed that the gambier extract exerted higher antibacterial activity against gram-negative bacteria E. coli and Salmonella sp. than a gram-positive bacterium, Staphylococcus aureus [16]. Other studies have also confirmed that gambier extract has anti-inflammatory [17], anticancer [18], and antidiabetic [19] properties.
Oral diseases, such as periodontal disease, dental caries, oral mucosal lesions, and tooth loss, are becoming major health problems worldwide [20]. Dental implants are an alternative treatment to replace teeth lost due to periodontal disease, which has been increased due to their high success rate and comfort in dentures [21, 22]. However, implant failure can still occur, especially during the implant healing process. In this process, bone regeneration and healing occur between the implant and bone, which takes 3 months to more than 6 months [23]. However, bone implants (autograft and allograft) have some drawbacks, such as limited resources, extra-invasive surgery, and immunological rejection [24]. For these reasons, innovation is needed in agents that can effectively enhance the inflammation process in implant installation, bone formation, and regeneration.
In dentistry, many in vitro studies have evaluated the biocompatibility, toxicity, and efficacy of dental materials [25]. The selection of fibroblast cells for cytotoxic testing is due to fibroblasts being the most common cells in the pulp, which is the target of the chemical components tested [26]. Fibroblasts are cells that form the basis of connective tissue and help connective tissue derivatives, such as bone, cartilage, and blood, to differentiate [27]. Fibroblasts are known to function in response to inflammation by recruiting immune cells, producing cytokines and chemokines, and modifying tissues during wound healing [28, 29]. The synthesis of new tissue by fibroblasts is required to rebuild tissue in response to injury [30]. Fibroblast cells are required for periodontal ligament regeneration because they are the first cells to come into touch with the irrigant when it extrudes from the apical foramen [31]. NIH-3T3 is one of the cells that is often used in viability and toxicity tests [32]. For example, in dentistry, NIH-3T3 cells are used as a substitute for human gingival fibroblasts. NIH-3T3 cells are a continuous cell line that has a highly reproducible growth rate and biological response [33, 34].
Due to its bioactivity, numerous studies have investigated natural extract in dental treatments, such as dental caries, periodontal disease, pulp pathology, dental restorative, and oral cancer [7]. Moreover, a previous study investigated the application of gambier extracts, such as formulations in mouthwash and toothpaste [9]. The antioxidant, anti-inflammatory, and anti-bacterial properties of gambier trigger further research in vitro and in vivo. Therefore, further research is needed on fibroblast cells. In addition, the potential of gambier extract also needs further research. Based on our knowledge, no study evaluated the cytotoxic activity of gambier extract on NIH-3T3 cells. In this study, we evaluated the cytotoxic activity of Uncaria gambir or gambier ethyl acetate extract (GEE) on NIH-3T3 cells. The null hypothesis was tested that GEE on various concentrations and incubation periods does not affect the cell viability of NIH-3T3. Furthermore, the cytotoxic activity was evaluated using the MTT method. We measured the cell viability and cytotoxic parameter by CD50 value.
2. MATERIALS AND METHODS
2.1. Materials
The materials used for the cytotoxicity test were obtained from ethyl acetate extract of gambier (Uncaria gambir Roxb.) leaves. The gambier leaf was obtained from Padang, West Sumatra, Indonesia. The chemicals used were dimethyl sulfoxide (DMSO) (Sigma-Aldrich, D8418), 70% alcohol, the Dulbecco's Modified Eagle's Medium (DMEM, Gibco: 1195065), trypsin-EDTA (Gibco: 25200072), phosphate buffer saline (PBS, Gibco: 10010049), Fetal Bovine Serum (FBS, Gibco: 10270206), 1% penicillin/streptomycin (10.000 Unit/mL) (Gibco: 1514022), trypan blue and MTT reagent 3-(4,5-dimethylthiazol-2-il) -2,5-diphenyltetrazolium bromide (M2128, Sigma-Aldrich), and reagent stopper.
2.2. Preparations of Uncaria gambir Extract
For extraction, maceration methods were employed with methanol as a solvent for 3×24 hours. Then, the solvent was evaporated using a rotary evaporator, and an extract paste was obtained. The paste was diluted with water and partitioned with a separating funnel. Partition split was done twice. The first partition was separated with n-hexane, and the second partition was done using ethyl acetate [35, 36]. The gambier ethyl acetate extract (GEE) was used for the next test. GEE was used in ten concentrations, namely 1000, 500, 250, 100, 50, 25, 10, 5, 2.5, and 0 ppm in DMSO. The 0-ppm was used as a control.
2.3. Cell Culture
Mouse embryonic fibroblast cells (NIH-3T3 cell line, ATCC CRL-1658) were cultured at Cell Culture and Cytogenetic Laboratory, Padjadjaran University, Bandung, Indonesia. Cells were cultured in DMEM supplemented by 10% FBS and 1% penicillin/streptomycin. The cell was incubated at 37°C in a humidified atmosphere with 5% of CO2 [37].
2.4. Cytotoxic Assay
The cytotoxic activity of GEE was evaluated by MTT (3-[4.5-dimethylthiazol- 2-yl]-2.5 diphenyl tetrazolium bromide) at Cell Culture and Cytogenetic Laboratory, Padjadjaran University, Bandung, Indonesia. An 80% cell confluency was seeded in a 96-well plate with 5×103 cells per well. GEE at various concentrations (1000, 500, 250, 100, 50, 25, 10, 5, 2.5, and 0 ppm) was added to each well. The plates were incubated for 24-, 48-, and 72 hours. The medium was removed, and the MTT (5 mg/mL in PBS) was added; then, the plates were incubated for 2-4 hours. The MTT crystals were homogenized in 100 μL DMSO to each well. Absorbance was measured at 570 nm using a spectrophotometer (Spectra MAX PLUS, Molecular Devices, Sunny vale, CA, USA) [38-40].
2.5. Statistical Analysis
The data were presented as the mean ± standard deviation. Statistical analysis was performed with SPSS software 20.0 version (SPSS Inc., USA). The obtained data were analyzed by one-way ANOVA test followed by Tukey HSD post hoc with p<0.05. Pairwise comparison was analyzed using ANOVA paired sample T-test with p<0.05. The CD50 value was measured by SigmaPlot™ 13 software (Systat Software Inc., San Jose, CA, USA).
3. RESULTS
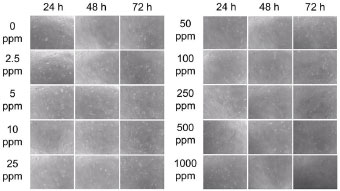
In this study, we investigated the cytotoxic activity of the gambier extract on NIH-3T3 cells using an MTT assay. The effect of GEE on the cell viability of NIH-3T3 at 24-, 48, and 72 hours can be seen in Figs. (1-3), respectively. The effect of GEE on the microscopic morphology of NIH-3T3 cells at 24-, 48-, and 72 hours can be seen in Fig. (4).
The highest GEE concentration (1000 ppm) showed the lowest cell viability percentage significantly to 0 ppm for all time treatments. The CD50 for all incubation periods can be seen in Table 1. CD50 of GEE in 24-hour treatment was 578.03 ppm. Treatment of 48 hours for GEE showed the lowest cell viability percentage significantly to 0 ppm at 1000 ppm and 500 ppm. GEE at 1000 ppm showed the smallest cell viability value, which was 30.14%, and GEE cell viability at 500 ppm was 40.43%. The CD50 of GEE in 48-hour treatment was 488.63 ppm.
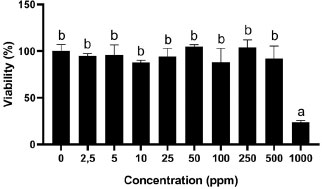
*Three replicates were used to determine the cytotoxicity of each concentration. Data were presented as means ± standard deviation. Different letters (a, b) show significant differences among concentrations with P<0.05 based on ANOVA Tukey post hoc.
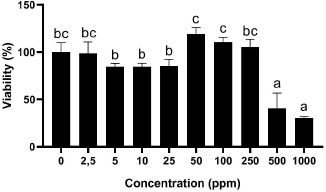
*Three replicates were used to determine the cytotoxicity of each concentration. Data were presented as means ± standard deviation. Different letters show significant differences among concentrations with P<0.05 based on ANOVA Tukey post hoc.
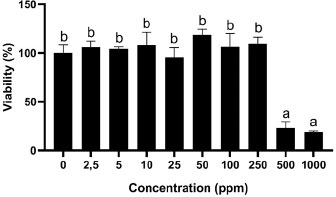
*Three replicates were used to determine the cytotoxicity of each concentration. Data were presented as means ± standard deviation. Different letters show significant differences among concentrations with P<0.05 based on ANOVA Tukey post hoc.
| Incubation Periods (Hours) | CD50 (ppm) |
|---|---|
| 24 | 578.03 |
| 48 | 488.63 |
| 72 | 470.70 |
Treatment of 72 hours for GEE showed the lowest cell viability percentage significantly to 0 ppm at 1000 ppm and 500 ppm. GEE at 1000 ppm showed the smallest cell viability value at 19.02%, and GEE cell viability at 500 ppm was 23.03%. The CD50 of GEE in 72-hour treatment was 470.70 ppm. Based on (Figs. 1-3), the longer treatment time demonstrated lower cell viability, especially at high concentrations (1000 ppm). Moreover, 500 ppm of GEE showed a toxicity effect on NIH-3T3 cells after 48- and 72 hours of treatment.
The cell viability % in 24-, 48-, and 72-hour incubation periods for each concentration were in accordance with the density of the cell (Fig. 4). The 48- and 72-hour incubation periods (Fig. 4) showed a decrease in density of cells, especially after 500 ppm of GEE. Analysis based on incubation periods is shown in Table 2. No significant difference was found among 24-, 48-, and 72-h incubation periods of GEE treatment on cell viability % of NIH-3T3.
4. DISCUSSION
In the present study, the cytotoxic of GEE was evaluated on NIH-3T3 cells by the MTT method. The results warrant the rejection of the null hypothesis that the addition of GEE does not affect the viability of NIH-3T3 cells. The results showed an increase in the viability of cells with the addition of 2.5-250 ppm GEE compared to 0 ppm GEE treatment at 24-, 48-, and 72-hour incubation periods. The result also showed that GEE at 1000 ppm was toxic to NIH-3T3 cells on 24-hours incubation periods with a significant decrease in the cell viability percentage <80%. In addition, 500 ppm of GEE was toxic to NIH-3T3 cells at 48-hour and 72-hour incubation periods.
The GEE at 1000 ppm showed the lowest cell viability, which was 23.83%. The value of cell viability in 24-hour treatment is not based on the concentration of GEE. There was an increase in cell viability at concentrations of 50 ppm and 250 ppm compared to 0 ppm GEE, with cell viability of 104.75% and 104.05%, respectively. A previous study investigated the cell viability of U. gambir extracts on RAW 264.7 cells. It was found that 1, 10, and 100 μg/mL (ppm) of U. gambir extract for 24 hours were not toxic to RAW 264.7 cells. The cell viability increased from the control [41]. This result was in line with the present study, which demonstrated that the addition of U. gambir extract in the range of 2.5 to 100 ppm for 24-hours incubation periods was non-toxic to the cell. Furthermore, another study showed that 250 and 500 μg/mL concentrations of U. gambir extract were non-toxic to human gingival fibroblast cells (HGFC). Moreover, U. gambir extract at 500 μg/mL showed the highest cell viability than 250 μg/mL concentration of U. gambir extract [12]. These results were in line with the present study, in which 10-500 μg/mL (ppm) of U. gambir extract was non-toxic to the cell. GEE for 24 hours at 2.5-500 ppm was non-toxic to the NIH-3T3 cell with cell viability % of up to 80% (Fig. 1).
In our study, GEE has shown cytotoxic activity in the concentration of 1000 ppm for all incubation periods, while GEE at 500 ppm demonstrated cytotoxic activity after 48- and 72-hour incubation. GEE at 500 ppm and 1000 ppm were toxic to NIH-3T3 cells by decreasing the cell viability significantly to 0 ppm. Similar to the results of the treatment time of 24 hours, the value of cell viability at 48 hours of treatment was not based on the concentration of GEE. There was a significant increase in cell viability at concentrations of 50 ppm, 100 ppm, and 250 ppm compared to 0 ppm of GEE. GEE concentrations of 50 ppm, 100 ppm, and 250 ppm indicated cell viability values of 119.30%, 110.60%, and 105.14%, respectively.
U. gambier has long been used as an alternative medicine traditionally. The main component of U. gambir extract, which is well known, is catechin [42]. The previous study showed that catechin at 1, 10, and 100 μM increased the proliferation of cells significantly than control NIH-3T3 cells in an oxidative stress state [18]. Moreover, the ethyl acetate extract of U. gambir showed the highest total phenolic content, followed by catechin and tannin content, compared to water and ethanol extract [6]. Concerning this, the increase in cell viability in U. gambir treatment can be caused by the high content of catechin.
| - | Mean | Std. Deviation | Std. Error Mean | 95% Confidence Interval of the Difference | t | df | Sig. (2-tailed) | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| 24 h – 48 h | 2.64443 | 21.39264 | 3.90574 | -5.34371 | 10.63258 | 0.677 | 29 | 0.504 |
| 24 h – 72 h | -0.47779 | 25.87204 | -10.13857 | -10.1385 | 9.18299 | -0.101 | 29 | 0.920 |
| 48 h – 72 h | -3.12222 | 15.84357 | -9.03831 | -9.03831 | 2.79386 | -1.079 | 29 | 0.289 |
Cytotoxic tests are used to evaluate the biomaterial's extract reaction toward cell culture [43]. The present study evaluated the cytotoxic activity of gambier extract using the MTT method by measuring the cell viability. The parameters of cytotoxic activity in this study were cell viability % and CD50 value. In cytotoxic activity, the CD50 value is a concentration of the sample that inhibits the 50% proliferation of the cell. The higher the CD50 value, the less toxic the sample is to the cells [44]. In this study, the CD50 value of GEE decreased depending on the incubation period. The CD50 value of GEE at the 24 hours incubation period was 578.03 ppm, the CD50 at 48 hours was 488.63 ppm, and at 72 hours, it was 470.70 ppm (Table 1). GEE has a CD50 value > 100 ppm. It was indicated that GEE was non-toxic to NIH-3T3 cells.
This study showed that the use of GEE was not toxic to fibroblast cells. GEE is a potent natural product in bone formation and regeneration therapy. The potential of GEE on cytotoxic activity needs to be further studied toward more periodontal cells to explore the bioactivity of gambier extract and also in in vivo study to observe the bone healing process. Moreover, additional testing is required to amplify the GEE as an adjuvant bone formation and regeneration agent. Hence, our study can be useful in providing new information or supporting information for further study of U. gambir bioactivity.
CONCLUSION
The present study showed that GEE at 1000 ppm affected the cell viability of NIH-3T3 in all incubation periods (24-, 48-, and 72 hours). Moreover, GEE at 500 ppm concentration in 48- and 72-hour incubation demonstrated cell viability of < 80%.
LIST OF ABBREVIATIONS
| ANOVA | = Analysis of Variance |
| CD | = Cytotoxic Dose |
| DMEM | = Dulbecco's Modified Eagle's Medium |
| DMSO | = Dimethyl Sulfoxide |
| FBS | = Fetal Bovine Serum |
| GEE | = Gambier Ethyl Acetate Extract |
| HGFC | = Human Gingival Fibroblast Cells |
| IC | = Inhibitory Concentration |
| PBS | = Phosphate Buffer Saline |
| SPSS | = Statistical Package for the Social Sciences |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals and humans were used in the studies that are the basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIAL
The data that support the findings of this study are available from the corresponding author, [F.P], on special request.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest regarding the publication of this article.
ACKNOWLEDGEMENTS
The authors would like to thank Cell Culture and Cytogenetic Laboratory, Eyckman Laboratory Padjadjaran University, Bandung, Indonesia, for providing facilities and valuable assistance.


