All published articles of this journal are available on ScienceDirect.
Electronic Apex Locators and their Implications in Contemporary Clinical Practice: A Review
Abstract
Background:
The apical extent of instrument termination and final root-filling procedures have been found to be key prognostic factors in relation to the outcome of root canal treatment. The precise location of this termination point has always been a challenge in clinical endodontics. Until the introduction of contemporary electronic apex locators (EALs), conventional radiography was traditionally used to determine the working length. Since their inception more than 50 years ago, EALs have drawn a great deal of attention.
Objective:
The literature pertaining to these devices is saturated yet inexhaustive and controversial. While most reviews try to summarize this topic entirely, the scope of the subject makes this close to impossible. Most systematic reviews report a lack of high-quality evidence, making it impossible to reach a meaningful conclusion. This review of literature focuses on electronic apex location and its accuracy, specifically in relation to variables in the clinical setting that a practitioner might encounter while using this device.
Methods:
Electronic database searches were undertaken using a combination of key search words to find relevant studies about EALs.
Results:
The PubMed (MEDLINE) search engine was used to find studies published in the English language with no restrictions for time. Articles that were found to be most pertinent were chosen and included in the review.
Conclusion:
EALs are indispensable to the practice of endodontics; however, the adjunct use of radiographs remains a reasonable practice.
1. INTRODUCTION
The success rate of conventional root canal treatment (RCT) has improved in recent years compared to previous decades. This is especially true when basic RCT guidelines are followed. As thorough cleaning, sufficient shaping, and complete filling of the root canals cannot be achieved without the establishment of working length (WL), accurate root canal length determination is critical to the success of RCT. Another key aspect to remember is that once the canal length is determined, harm to the periapical tissues and procedural errors can be prevented by restricting instruments and filling materials within the root canal system [1]. Radiographs are still standard procedure for determining WL; however, the accuracy of canal length is difficult to attain because the apical constriction (AC) cannot be recognized. Furthermore, there are too many variables in this technique, such as angulations and exposure, that can affect the final image, resulting in errors [2, 3]. As a result, in addition to radiography measures, the use of apex locators (EALs) for determining WL has gained significant importance. For more than 50 years, EALs have been utilized in the clinical setting as a tool for determining WL. When connected to a file, these devices detect the point at which the file exits the canal and enters the periodontium. Many of the issues associated with radiographic measurements are averted by using this electronic method. The length measurement of the root canal to the end of the apical foramen, rather than the radiographic apex, is perhaps its most significant advantage over radiography [4].
1.1. The Significance of WL
Grove [5] stated that “the proper point to which root canals should be filled is the cementodentinal junction (CDJ) and that the pulp should be severed at the point of its unification with the periodontal membrane”. The CDJ is an anatomical and histological landmark that marks the point at which the pulp ends and the periodontal ligament begins. Biomechanical preparation techniques of the root canal aim to make use of this potential natural barrier [6]. According to an in vivo research [7], the most favorable histological conditions occurred when endodontic procedures, including preparation and filling, terminated short of the apical constriction (AC). Instrumentation beyond the apical foramen should be avoided, as it may cause postoperative pain and sensitivity. Filling with extrusion of gutta-percha and sealer beyond the radiographic apex induces an inflammatory reaction, thereby reducing the success of RCT. Short WL, on the other hand, will most likely have no effect on post-treatment outcomes unless necrotic, in which infected tissues remain in the apical area, resulting in adverse implications [8, 9]. The failure of RCT is mainly attributed to the persistence of microorganisms within the root canals. According to present epidemiological evidence, obturation within 0-2 mm of the radiographic apex is associated with the most favorable outcome. Consequently, the outcome is adversely affected when the canals are overfilled or underfilled, especially in cases of preexisting infection [10, 11]. The conundrum clinicians face is how to precisely establish the apical limit of preparation, the ‘WL’, to achieve optimum success. This endeavor is made more difficult by the differences in the architecture of tooth apices that occur with age and tooth type.
The use of EALs alone or adjunct to radiographs is a topic of debate amongst clinicians. According to Saad and Al-Nazhan [12], successful RCT is dependent on correct WL measurement and can be accomplished with an EAL alone. However, The European Society of Endodontology [13] recommends combining radiographic and electronic approaches to determine WL. A study by Kim et al. [14] showed this recommendation to be true where the Root-ZX alone identified the AC with a precision of 84%, which increased to a staggering 96% when root-ZX was combined with radiographs.
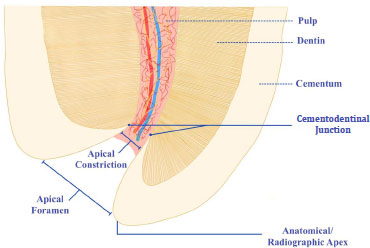
1.2. Anatomy of The Apical Region
An understanding of apical anatomy is integral to fully appreciating the concept of WL. This requires an acknowledgment of a few fundamental elements of the apical region. (Fig. 1) illustrates these as the anatomical apex, the apical foramen (major foramen), the AC (minor foramen), and the CDJ [15]. The AC, when present, is the narrowest region of the canal with the smallest blood supply diameter. The resultant wound site after preparation up to this landmark will therefore be small, and the healing conditions will be optimal [7]. The AC's location and its relationship to the CDJ differ substantially from root to root. The CDJ is very uneven, and on one root wall, it can be up to 3 mm higher than the opposing wall [16].
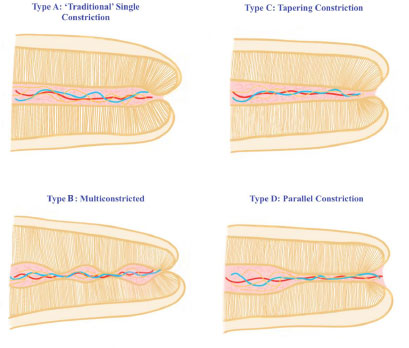
Many studies [15, 17, 18] have revealed that the apical foramen does not necessarily correspond to the tooth's anatomical apex and can be found to one side of the anatomical apex, occasionally even at distances up to 3 mm. Kuttler [15] measured the apex-to-foramen distances and found them to vary amongst young and old groups (0.48 mm and 0.60 mm, respectively). A study by Dummer et al. [19] showed that the average foramen to apex distance in anterior teeth was 0.36 mm, which agreed with previous studies by Green [17, 20], where this distance was measured to be 0.3 mm in anterior teeth and 0.43 mm in posterior teeth. For all tooth types, the distance from the AC to the foramen had an estimated average of 0.8 mm in the older group, while in the younger group, it was found to be 0.5 mm [15, 19, 21]. The foramen to apex distance is typically greater in posterior and older teeth when compared to anterior and younger teeth. The presence or absence of apical disease, the age, and the kind of teeth studied, as well as the inconsistency of the minor foramen, may all contribute to the range of measurements obtained from anatomical studies. The AC was classified into 4 types (Fig. 2) by Dummer et al. [19], and they hypothesized that using this concept would result in under preparation in type B and over-preparation in type D. Consequently, based on the available studies, at best, radiography can provide an approximation of the histological configuration. Averages utilized for the AC from the radiographic or anatomical apex, while clinically desired, may result in overfilling or underfilling of the root canal systems. The frequent practice of estimating WL to be 1-2 mm short of the anatomic apex observed on a radiograph has resulted from using these averages and assuming that the CDJ occurs at the AC [22].
2. METHODOLOGY
Information about electronic apex locators (EALs) was gathered using the PubMed (MEDLINE) search engine. There were no restrictions on time. However, only studies published in the English language were included. To search for relevant articles, the PubMed dataset’s Boolean option was used using a variety of terms, including “apex locators” AND “root canal OR working length”. Searches were carried out on Google Scholar, again with no time restriction, to acquire more articles. Using this process, 726 articles were obtained. Checking the reference lists of pertinent papers yielded even more studies. Articles that were found to be most significant in relation to the rationale of the review were chosen and are included in the reference list.
3. EVOLUTION OF EALs
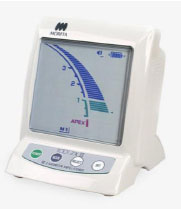
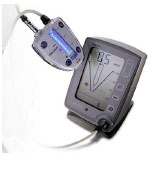
Custer [23] was the first to investigate the use of an electronic method to determine the length of the root canal. Suzuki revisited this concept in 1942, when he investigated the flow of direct current through the teeth of dogs [24]. Consistent electrical resistance values were recorded between an electrode in contact with the oral mucous membrane and an instrument within the root canal. Therefore, he hypothesized that this technique might be used to quantify canal length. Concerning these fundamental concepts, specifically, the consistent electrical resistance of 6.5 kΩ between the periodontium and mucosal membrane, Sunada [25] created a simple device that measured canal length using direct current. This principle was true irrespective of the age of the person or the type of teeth. The categorization of EALs has frequently appeared to be perplexing to clinicians. EALs are typically classified according to the generation they belong to, which in turn is based on their working principles, with each generation having its own advantages and disadvantages. Table 1 provides a brief summary of the different generations of EALs.
| Generation | 1st | 2nd | 3rd | 4th | 5th | 6th |
| Photo |  |
 |
 |
 |
 |
 |
| Brand name | The Root Canal Meter (Onuki Medical Co., Tokyo, Japan) | Sono-Explorer (Hayashi Dental Supply, Tokyo, Japan) | Root ZX (J. Morita, Kyoto, Japan) | Element Dianostic Unit & Apex Locator (Sybron Endo, Anaheim, CA, USA) | Propex II (Dentsply Maillerfer, Ballaiques, Switzerland) | Raypex 6 (VDW, Munich, Germany) |
| Year of invention | 1969 | 1971 | 1991 | 2003 | 2003 | 2013 |
| Mode of action | Measure electrical resistance. | Measure electrical impedance. | Use two different frequencies at the same time in order to measure the difference or ratio between two currents. | Use two or more non-simultaneous continuous frequencies to measure the difference or ratio between two currents. | Measure the capacitance and resistance of the circuit separately. | Adaptive devices that have a steady algorithm for measuring the working length of the root canal depending on the canal’s moisture characteristics. |
| Strengths | digital read out audible indication detects perforation may incorporate pulp tester |
does not require a lip clip no patient sensitivity |
have powerful microprocessors that give precise readings. easy to operate audible indication can operate with electrolytes analogue read out |
accurate determination of the apical terminus | error free in many conditions (with blood, irrigants) digital read out graphic illustration may incorporate pulp tester |
precise measurement quick results immediately adapt to dry or wet canal |
| Weakness | requires dry field patient sensitivity requires calibration requires good contact with lip clip many significantly longer |
no digital read out difficult to operate problems with dry canals as well as electrolytes in the canals |
requires lip clip | low accuracy in working in wet canals chances of short circuit |
difficulty in working in dry canals and require additional wetting |
4. OTHER ASPECTS OF EAL USE
Some innovative and atypical uses for EALs have been reported in the literature. Suspected periodontal or pulpal perforations are reliably detected by all modern EALs to clinically acceptable limits. Furthermore, they can discern between small and large perforations [26, 27]. The instrument completes a circuit when it passes through a patent perforation and is indicated on the EAL as being beyond the ‘apex’ [28]. Justy II has been reported to be incapable of detecting simulated vertical fractures, but it has been found to be reliable when assessing simulated horizontal fractures [29]. The EAL will detect any connection between the periodontal ligament and the root canal (cracks, fracture, and external or internal resorption) and can therefore serve as a brilliant diagnostic tool.
For a short period, electrically conductive gutta-percha gained favor in hopes of reducing radiographic exposure and preventing under-extension and over-extension of filling materials. According to Rivera and Seraji [30], the accuracy of these cones with apex location measurements proved to be clinically unacceptable.
EALs with multiple functions are becoming increasingly prevalent, and many of them have vitality testing capabilities. Handpieces integrated with EAL function greatly assist in the mechanical preparation of the root canal system and have become popular as this arrangement allows for improved workflow, efficiency, and reliability, at the same time producing outstanding results with the same precision as stand-alone devices [31]. Some integrated rotary motors are the Endy 7000 and Dentaport ZX. A study that tested the ability of MM Control and Root ZX II to maintain the apical extent during mechanical instrumentation showed them to be extremely accurate when used up to 0.0 mm from the apical foramen. However, these devices presented reduced accuracy when limited to -1.0 mm from the apical foramen. Furthermore, the electronic apex location function of Root ZX II was found to be more accurate, with the MM Control resulting in a greater proportion of overestimated readings [32].
5. EALs AND CLINICAL VARIABLES
5.1. EALs and Cardiac Pacemakers
There is an increased prevalence in the number of patients with cardiovascular implantable devices visiting dental offices. These are small electronic devices that operate on lithium batteries and are surgically introduced near the left clavicle underneath the skin [33]. They detect arrhythmias by monitoring the heart rate continuously by means of insulated, flexible leads that traverse through the veins to the heart [34, 35]. Contemporary implantable pacemakers are equipped with hermetically sealed covers, bipolar modes, rejection circuits, and filters, which allow significant protection against any electromagnetic interference [36]. There is, however, a potential for electromagnetic interference, which is why manufacturers of these devices explicitly caution clinicians regarding their usage in these cases [37].
Since there are several different kinds of EALs available in the market, it is important to know how they influence these implantable devices. A few authors have found that electronic dental equipment can interfere with the function of these devices [38]. However, there are others who have concluded that there is no significant effect of dental equipment on cardiovascular implantable devices. A bench top test was carried out by Garofalo et al. [39] using third-generation EALs from 5 different companies. They concluded that all EALs barring Bingo 1020, did not trigger any kind of interference or inhibition with the normal function of pacemakers. Therefore, they recommended the safe use of these devices in patients with implanted pacemakers. As this was an in vitro study rather than a clinical trial, the researchers suggested that a consultation with the patient’s cardiologist might be prudent before initiating any treatment. In addition, several in vitro studies have found that EALs did not interfere with the performance of pacemakers, indicating that they might be utilized safely in such patients [33, 35, 40-42]. A review by AlRahabi et al. [43] established the safe use of EALs in patients with pacemakers if all recommended precautions are taken, such as keeping these appliances a minimum of 10-20 cm away from the patient. Nonetheless, it is still cautiously advisable to consult with the patient’s treating cardiologist.
5.2. EALs and Maxillary Sinus
The maxillary sinus is localized adjacent to the nasal cavity in close relation to the upper molars [44]. Inflammation of the maxillary sinus is known as sinusitis. A study reported that of 190 studied unilateral sinusitis cases, 70% were caused by odontogenic infections [45]. A leading cause is iatrogenic errors, which may induce sinusitis and infections. This includes endodontic procedures, such as biomechanical preparation and filling involving extrusion of irrigants, endodontic files, medicaments, and/or obturating materials into the sinus [46, 47]. Therefore, precautions should be taken during surgeries as well as for RCT of maxillary molars with roots close to the maxillary sinus [48]. Aksoy and Orhan [49] investigated maxillary first and second molars and discovered that in 77 and 84% of cases, respectively, at least one root protruded into or was in contact with the sinus. Gu et al. [44] studied the relationship of the posterior maxillary teeth to the floor of the maxillary sinus and found the molars to be closer than the premolars. The person’s age was found to have a substantial impact on the relationship between the position of the maxillary sinus floor and the roots of the maxillary molars.
Unfortunately, the reliability of EAL function in cases of teeth with root apices close to the maxillary sinus is a topic not extensively studied. A study by El Hachem et al. [50] showed a significant difference in the actual WL versus the estimated WL of the palatal roots that were in contact with the sinus as compared to the roots that were not contacting the sinus. In these palatal roots, the actual WL was calculated to be significantly shorter than the estimated WL. Previous studies [51-53] confirmed these results of over-estimated measurements in 3.1-, 2.56-, and 7.9% of cases when using the Root ZX. It is hypothesized that the levels of impedance differ between roots that are in contact with the maxillary sinus as compared to those which are not. This is critical as this overestimation poses the risk of over-instrumentation, and inaccurate determination of WL could result in the extension of bacteria, intracanal solutions, endodontic files, and root canal fillings into the sinus [54]. Most importantly, chronic sinusitis can be caused by the iatrogenic introduction of infected necrotic debris into the sinus [55]. This is particularly applicable to those clinicians who solely rely on their EAL to perform RCT. Therefore, it might be prudent to combine periapical radiographs with EALs in such cases to prevent possible complications and allow the success of the RCT in the long run.
5.3. EALs and Open/Wide Apical Foramen
Open apex is a term frequently used to refer to an unusually wide apical foramen. Consequently, in these cases, achieving a preparation of the classic apical ‘stop’ is quite difficult, if not impossible. Authors consider different minimum ISO sizes and therefore have different definitions, which include ISO 100 [56], ISO 80 [57, 58], ISO 60 [59, 60], ISO 45 [61], and ISO 40 [62]. In the literature, there is little available information concerning the anatomy of incomplete apices. Friend (1966) described 3 types (divergent, parallel, tapering) of open apices when the radiographic analysis was carried out of immature permanent teeth in 7.5 -15-year-olds [57]. Several laboratory studies have shown that for AC diameters of up to ISO 60, the usage of file sizes 10 and above for electronic length, determination did not affect the accuracy of Root ZX [62-64]. However, when employed in cases of AC of ISO 100, the Root ZX proved to be unreliable, resulting in length errors that ranged between 0 and 1.03 mm on average. It was also unclear whether more reliable readings were obtained with the use of better-fitting files [62].
A group of researchers who studied the Root ZX found that upwards of size 80, there was a measurement inaccuracy of 2.24- and 0.88 mm when sizes 10 and 80 K-files were used, respectively. In the same study, the authors demonstrated that the Apit 7 showed the largest measurement inaccuracies of 2.84- and 1.61 mm. In this study, the Root ZX demonstrated a significantly better performance than the other 3 EALs tested [65]. Using the Root ZX, Herrera et al. [66], in their laboratory study, found that the device was accurate for apical sizes of 60, irrespective of the file size used to determine the WL. However, for apical sizes of 70 and above, better-adapting files with a minimum of ISO 45 were required to obtain reliable results. Their results were promising because they showed that the preciseness was between 95–99% for all diameter sizes studied.
Alternative methods of length determination for teeth with open apices have been investigated in many studies. A novel tactile technique was studied by a group of researchers which involved the advancement of a size 30 paper point into the canal until some resistance was felt. It was found to be comparable to the accuracy of radiographic length around 95% of the time, irrespective of the size of the apex or the presence of apical pathology. Therefore, they concluded that when this method was utilized, a WL radiograph was not required. A drawback, however, is the underestimation of the WL in cases where periapical soft tissues extend into the canal [67]. There is a requirement for a precise definition and consensus on what constitutes an open apex, as well as guidelines that allow clinicians to achieve successful outcomes in these cases. Therefore, as per the best evidence available, supplementary techniques must be used in conjunction with EALs and radiographs. It should be noted, however, that there are no long-term studies on these techniques.
5.4. EALs and C-shaped Canals
A “C-shaped canal” was first termed by Cooke and Cox [68] and is reported to occur because of the fusion of the distal and mesial roots, which could be either on the lingual or the buccal aspect of mandibular molars. These canals usually have single ribbon-shaped orifices with arcs greater than 180o. However, below the level of the orifice, a wide range of anatomical variations can be present. In other words, it is not always a continuous C-shape from the orifice to the apical foramen [69]. Cheung et al. [70] studied the apical anatomy of mandibular second molars with C-shaped canal systems using micro-CT and stereomicroscopy. They found that most teeth had either 2 or 3 canals, and nearly a third of them had 2 or 3 apical foramina. The presence of accessory foramina had a prevalence of about 48%. These complexities in the anatomy of the area near the apical region imply that endodontic procedure, including the establishment of WL, cleaning, shaping, and filling of the C-shaped canal system, poses great challenges for a clinician [71].
A study by Jafarzadeh et al. [72] using the Root ZX mini EAL showed that the radiographic method resulted in a 20% overestimation of the WL. While with the electronic method, the WL overestimation was at 7.3%. The results of this study showed that when establishing WL in C-shaped canals, the EAL was more precise than conventional radiography. Consequently, this could possibly reduce the risk of overestimation in these canals. Based on this study, this difference could be important clinically. Like with any laboratory study, caution needs to be exercised, and these results must be verified by clinical studies. There is a dire need for more in vitro and in vivo studies to make relevant recommendations.
5.5. EALs and Retreatment
It is crucial in cases of RCT to precisely determine the root canal length to remove all fillings and debris and establish patency. Ng et al. [73] found that the odds of success were reduced by 12% for every 1 mm of the canal short of the terminus that remained un-instrumented. According to Bergenholtz et al. [74], successful treatment in teeth with overfilled root canals was only achieved in 36% of the cases. However, when accurate length determination was achieved during retreatment, this success rate increased to 62%. Previous research on retreatment has shown that the complete removal of residual materials, including debris, organic tissues, solvents, calcium hydroxide, sealers, and gutta-percha from the canals, is highly unlikely [75, 76]. According to studies by Rivera and Seraji [30], Ibarrola et al. [77], and Uzunoglu et al. [78], the accuracy of EALs was negatively affected by these residual materials [30, 77, 78]. Solvents used in retreatment operations were shown to have an unfavorable effect on the accuracy of these devices due to their electrical conductivity [75, 79].
Goldberg et al. [80] investigated the electronic length determination accuracy of 3 EALs (NovApex, Propex, Root ZX) to the actual WL during RCT. The study revealed that all 3 EALs were accurate within 0.5 mm at 80-, 85-, and 95%, respectively. Within 1 mm, NovApex, Propex, and Root ZX were found to be 95-, 95-, and 100% accurate, respectively. Tufenkci and Kalayci [81] found accuracy values for Dentaport ZX, Propex Pixi, and iPex II to be 90-, 86.6- and 86.6%, respectively, at ±0.5 mm margin range and 96.7-, 93.3- and 93.2% at ±1 mm margin range, respectively. However, none of these values were statistically significant. Ultimately, clinicians must be aware that materials that remain in the canal during retreatment could influence the function of EALs, resulting in overestimation or underestimation of the WL, and that a supplemental radiograph is recommended when in doubt.
5.6. EALs and Postoperative Pain
A common complication of RCT is postoperative pain, which has an incidence that ranges from 3- to 58% [82]. An acute inflammatory reaction can result from mechanical, chemical, and/or microbial injury to the pulp and/or periradicular tissues during RCT. Changes in the local environment, the release of inflammatory mediators, and an increase in tissue pressure, all contribute to postoperative pain [83]. According to Sjogren et al. [11], precise WL determination is one of the factors that influence the incidence of post-treatment pain. When a WL falls short of the AC, it results in the likelihood of pulp tissue remnants, insufficient debridement, and subsequent underfilling of the root canal systems, which can all cause prolonged pain. Apical perforation and overfilling of the canal, on the other hand, are the result of a WL established beyond the AC, which increases post-treatment pain and can defer or even prevent healing [84].
A study [85] that sought to ascertain whether using a radiograph or an electronic method of length determination influenced postoperative pain found no significant difference amongst the groups at any of the evaluated time points. Maximum pain was observed in the first 4 to 6 h, which is thought to be the time required for the anesthetic effect to wear off completely. These findings were in line with the results of earlier investigations [86, 87]. A previous study [88] yielded similar results where the severity of pain decreased to half on day one and less than 10% over the following seven days. Finally, whether WL is determined using an electronic approach or by means of conventional radiography, the degree of postoperative pain may not be affected.
5.7. EALs and Pulp/Periapical Conditions
Few publications exist in the literature, with conflicting data, when concerning the precision of EAL measurement and the status of the pulp and periapical tissues. In teeth with apical periodontitis, the structural intricacies of the apical third of the root canals could interfere with accurate EAL function. According to Akisue et al. [89] and Daniel et al. [90], pulp conditions had no effect on the precision of EALs. A study [91] that evaluated the accuracy of the Root ZX II in cases of apical periodontitis demonstrated that the presence of defects in the periapical area did not negatively affect the reliability or precision of the Root ZX II.
A study [92] comparing 4 EALs in relation to vital and necrotic teeth found no differences in electronic measurements for Justy II and Endox. However, for Root ZX and Endy, statistically significant differences were found. A few studies had contradicting conclusions. Pommer et al. [93] demonstrated that EALs were less accurate in teeth that exhibited pulp necrosis as compared to teeth with vital pulps. Serry et al. [94] investigated the accuracy of EALs in teeth with necrotic pulps with apical lesions and found that these devices were more accurate in teeth with vital pulps. Kuzminski [95] claimed that apical lesions negatively affected an EAL’s accuracy. Dunlap et al. [96] speculated that periapical resorption caused by the long-standing lesion could possibly result in the annihilation of the minor foramen, therefore affecting the accuracy of these devices. A systematic review [97], in which several relevant studies were analyzed to examine differences in electronic length measurements between vital and necrotic teeth, concluded that no significant difference exists in readings between the two pulpal conditions for any of the evaluated EALs.
5.8. EALs and Curved Canals
The accuracy of EALs may be affected by anatomical complexities, such as accessory canals, confluences, and isthmuses. As the degree of curvature increases, the error with WL measurements obtained with the radiographic method has been reported to increase [98]. It has also been shown that during different shaping procedures, the total length of curved canals varies [77, 99]. Even though outlining the accuracy of EALs in curved molars has clear clinical implications, most studies have focused on single-rooted teeth. Moreover, in a few studies that have investigated the accuracy of EALs in mandibular molars while analyzing the impact of canal curvature [100] and shaping procedures [77, 99], the Root ZX was the only device evaluated. A study evaluated the accuracy of Root ZX using a scanning electron microscope. When teeth with lateral foramina, which have acute curvatures at the apical third, were compared to teeth with normal apical foramina, the error in detecting the apex was much higher compared to the accuracy of conventional radiography to that of the Raypex 5 using teeth with curved and straight canals [101, 102]. According to the findings of this study, within ±0.05 mm of the actual WLs, the percentage of accurate electronic WL readings was 35% for curved root canals and 70% for straight canals.
A study [102] evaluated the effect of the degree of canal curvature on the accuracy of electronic readings compared to the actual canal length. The mild curvature group showed an accuracy of 95%, which differed significantly from the moderate and severe groups, which had an accuracy of 80%. Another study sought to determine the level of correlation between estimated WLs and electronic readings according to the degree of curvature. A total of 95% of samples in the moderate and severe curvature groups showed estimated WLs that were short of the apical foramen, whereas, in the mild curvature group, only 65% of samples had underestimated readings. It was hypothesized that the more curved the canal, the smaller its size and taper, thus the greater the frictional resistance encountered by the file. The accuracy of the device studied in detecting the apical foramen for all groups was 100% with a ±1 mm tolerance limit [103]. Piasecki et al. [104] used mandibular molars with curved mesial canals to study the accuracy of 3 EALs, including the Root ZX Mini, Apex ID, and CanalPro. They found that measurements at the 0.5 mark were accurate for all 3 EALs; however, using the 0.0 mark resulted in overestimated readings for the Apex ID. Amongst the anatomic variables evaluated in the study, the existence of a lateral foramen had a detrimental impact on the Apex ID reading at the 0.0 level.
CONCLUSION
It can be witnessed throughout the literature that there is no lone technique that is 100% accurate in determining endodontic WL. While modern EALs can determine canal length with more than 90% accuracy, they have their limitations. There exists an unequivocal consensus pertaining to the comparative accuracy between radiographic and electronic measurements of WL. Therefore, WL determination with EALs must be complemented by a radiographic control, thereby minimizing the radiation exposure of the radiographic determination method and the potential errors of electronic measurement. Conclusively, clinicians will be able to achieve foreseeable results through a thorough understanding of apical anatomy, accurate usage of EALs, and most importantly, using radiographs pragmatically.
LIST OF ABBREVIATIONS
| EALs | = Electronic Apex Locators |
| WL | = Working Length |
| AC | = Apical Constriction |
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


