All published articles of this journal are available on ScienceDirect.
Conservative Restorative Management of External Cervical Tooth Resorption: A Case Report
Abstract
Background:
External cervical resorption (ECR) is one of the subclassifications of external root resorption. The etiology of ECR is still unclear. In most cases, it is not evident and asymptomatic before the involvement of the pulp.
Case presentation:
This paper reports a case of ECR in the mandibular right first molar of a 24-year-old patient who presented with an asymptomatic pink tooth. A two-dimensional radiograph and Cone beam computed tomography (CBCT) showed cervical resorption penetrating at the cementoenamel junction level from the mesio-lingual aspect. It approximates the pulp horn coronally within the lingual dentinal wall.
Discussion:
The treatment of the case involved debridement of the resorptive defect and Mineral Trioxide Aggregate (MTA) was applied as a direct pulp capping, glass ionomer as a base, and the tooth was restored using composite resin restoration. After 6-months of follow-up, the radiographic examination showed healthy bone and periodontal structures with no evidence of periapical pathology.
Conclusion:
ECR is an aggressive and invasive lesion that acts silently, requiring early diagnosis and treatment for a successful outcome. The lesion size, location, and accessibility are the main factors affecting the successful treatment of ECR.
1. INTRODUCTION
Tooth resorption is the irreversible destruction of mineralized tissue (cementum and dentin) due to odontoclastic activity [1, 2]. Both primary and permanent teeth are susceptible to tooth resorption. Primary teeth are a physiological process that causes the teeth to exfoliate, allowing the permanent teeth to erupt [3]. However, in permanent teeth it is a pathological process that causes damage and/or loss of tooth structure [4]. Root resorption is classified as internal or external resorption based on its location on the root surface [3, 5]. External root resorption can be further subclassified, and one of the least understood and uncommon types is external cervical resorption (ECR) [5]. Cervical root resorption accounts for approximately 4% of all external root resorption cases [6]. Further, Heithersay came up with the term “invasive cervical resorption” to describe a cervical resorption's form with a more invasive and aggressive nature [7]. ECR may arise in the cervical region of the tooth, below the epithelial attachment, or in the coronal aspect of the bone [8].
Based on the literature, the etiology of ECR was differentiated into two putative mechanisms. The resorptive process is initiated either by sulcular microorganisms that activate the inflammation or by a benign proliferative fibro-osseous / fibrovascular condition in which microorganisms play no role but function as secondary invaders [9-11]. As yet, the etiology of ECR is unclear [12, 13]. Several studies reported various predisposing factors that may cause ECR. These factors include orthodontic treatment, dental trauma, internal bleaching, periodontal therapy, malocclusion, parafunctional habits, poor oral hygiene, intra-coronal restoration, surgical procedures (i.e., extraction of adjacent teeth, orthognathic surgery, etc.), eruption disorders, viral etiology, systemic diseases, and genetics [7, 14-16]. A few case studies reported a correlation between the feline herpes virus and ECR, demonstrating that it can be transmitted directly or indirectly from domestic, captive, and wild cats and cause human cervical resorption lesions [17-19]. Furthermore, systemic diseases such as Paget’s disease, Herpes Zoster infection, hyperparathyroidism, hypothyroidism [14], normocalcemic hypercalciuria, nephrolithiasis [20], neoplasia [21], hyperoxaluria, and oxalosis [22] have been proposed as possible causes of ECR.
ECR defects are quite challenging to diagnose and manage due to the inconspicuous clinical signs and symptoms and rapid progression [4, 23]. They are usually asymptomatic until they reach the pulp [24]. The resorption is mostly detected incidentally during a routine radiographic examination, and the only clinical sign is the presence of pink discoloration in the crown due to the high vascular granulation tissue [14, 25]. ECR is clinically classified based on the depth of the lesion into four classes [7], Class 1: a small lesion around the cervical region with shallow penetration into the dentin; Class 2: a well-defined resorptive lesion near the pulp chamber with minimal to no extension into radicular dentin; Class 3: resorption reaches the coronal third of the root; Class 4: resorption extends to the middle third of the root and beyond [7].
Many surgical and nonsurgical treatment modalities have been proposed to manage ECR [2]. There is no clear guideline for the treatment of ECR. Class 1 often needs a complete debridement of damaged tissue followed by a cavity restoration. [9,26] When the lesion is near the pulp, and there is a possibility of perforation while removing the granulation tissue, root canal treatment may be required in class 2, and class 3. [9,26] In addition, surgical intervention such as guided tissue regeneration, crown lengthening, and sometimes even intentional replantation is necessary if the lesion in the cervical area is inaccessible. [9,22,26] Some authors also consider orthodontic extrusion as an option with an apically positioned flap when the aesthetics are compromised. [9,26] In class 4 cases, the tooth is usually non-restorable, and extraction is the treatment of choice. [26] The correct diagnosis and comprehensive assessment of the size and location of the lesion are factors in determining the best treatment approach [2]. While reviewing the previous literature, case reports on the ECR are limited. Hence, this case report intended to describe a detailed, comprehensive method of assessment and management of ECR affecting the mandibular first molar utilizing various diagnostic tools such as cone beam computed tomography (CBCT), two-dimensional radiography, and histopathological analysis.
1.1. Case History
A healthy 24-year-old female visited the dental emergency room at Imam Abdulrahman bin Faisal University in Dammam, complaining that her tooth incidentally turned pink a month ago. Her medical and family history were noncontributory. Clinically, a pinkish discoloration was observed in the mesio-lingual cusp of the mandibular right first molar tooth #30 (Fig. 1). The patient did not report a history of trauma or tooth bleaching. The tooth had a history of deep occlusal restoration performed ten years ago. Also, the patient underwent orthodontic treatment five years ago. She had good oral hygiene with a healthy periodontal condition.
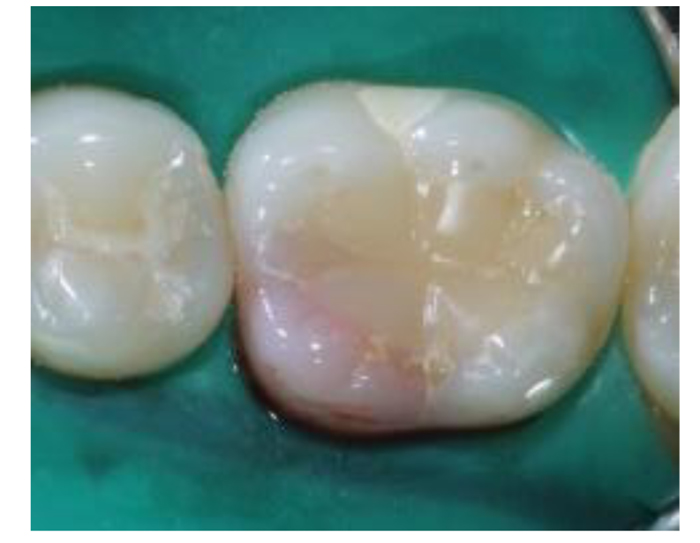
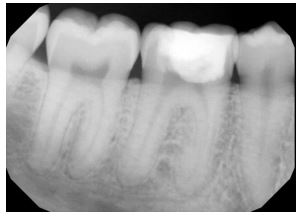
On examination, tooth revealed normal pulp sensibility testing to thermal and electric. It was non-tender to percussion, biting, or palpation. Also, there was no evidence of a sinus tract or swelling in the region. No mobility of the tooth was felt. Initial radiographic examination revealed a well-defined radiolucency in the mesial coronal part of the tooth #30, and no visible periapical radiolucency (Fig. 2A and B).
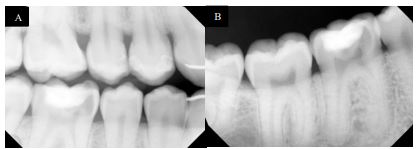
Cone beam computed tomography (CBCT) showed cervical resorption penetrating at the cementoenamel junction (CEJ) level from the mesio-lingual aspect. It approximates the pulp horn coronally within the lingual dentinal wall. No clear sign of periapical widening or periapical pathosis was observed. The supporting alveolar structures around the roots appeared to be intact. The external resorption pattern suggests invasive cervical root/crown resorption, Heithersay class II Fig. (3A-C).

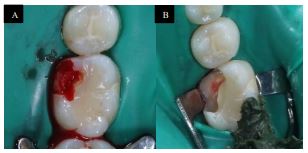
The treatment included debridement of the resorptive defect and pulp capping to restore the tooth. Under rubber dam isolation (Fiesta® Dental Dam, Natural rubber latex, COLTENE, US), a cavity preparation was made using high-speed carbide round bur (0.8 mm) and extended 2mm sub-gingivally to incorporate the resorptive defect. The granulation tissue was degraded mechanically using a stainless-steel spoon excavator (Nordent, Spoon Excavator #3 Double ended, Crown Medical & Dental, New Zealand). Pin-point pathological pulp exposure happened. The granulation tissue was submitted for histopathology analysis. Extensive saline irrigation was performed for the cavity until no granulation tissue was visible than with a small cotton pellet and pressure hemostasis was achieved (Fig. 4 A, and B). Then, Mineral trioxide aggregate (MTA) (White ProRoot MTA, Dentsply International Inc, Tulsa Dental, Johnson City, TN) was applied as a direct pulp capping, Glass Ionomer (PhotacTM Fil Quick AplicapTM,3M, ESPE, Germany) was used as a base, and the tooth restored using composite resin restoration (G-aenial™ Universal Flo, universal light-cured radiopaque flowable composite, Japan) (Fig. 5, 6). The future planned final restoration will be Ceramic Onlay.
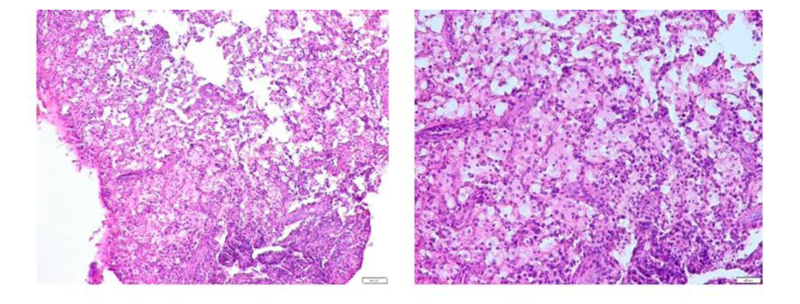
Microscopic examination revealed fibrous granulation tissue containing acute and chronic inflammatory cells, predominantly macrophages. Neutrophils, lymphocytes, and plasma cells were also seen (Fig. 7).
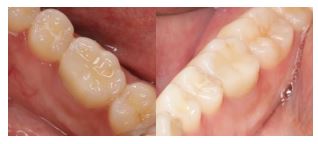
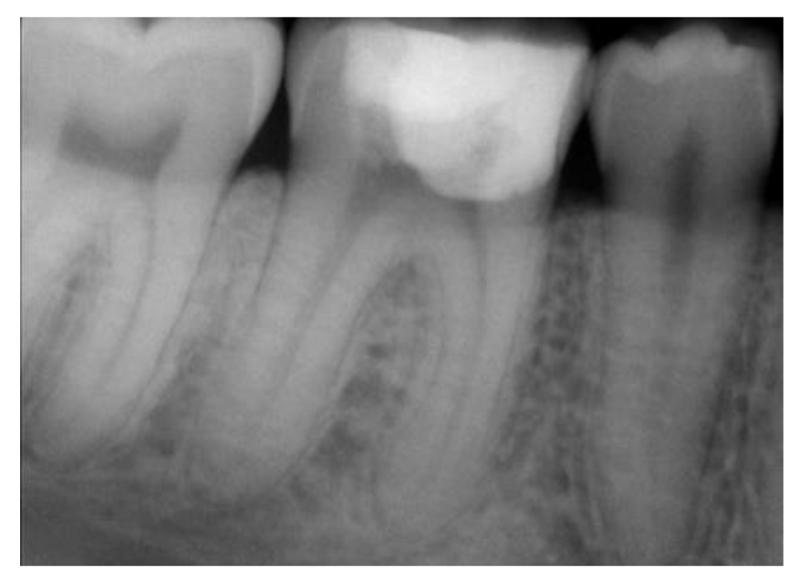
After a one-month follow-up, the patient was asymptomatic and there was no pain on palpation, biting, or percussion. A radiographic examination of the area at the 6-month follow-up showed healthy bone and periodontal structures with no signs of periapical pathology. (Fig. 8).
2. DISCUSSION
ECR is a rare form of external root resorption, which occurs when clastic cells damage the mineralized tissues in the cervical area of the tooth [1, 2, 8]. In the present case report, only a five-year history of orthodontic treatment was reported, with no other obvious predisposing factors such as trauma identified. Several case reports mentioned that their patients had previously undergone orthodontic treatment, suggesting that this could be a contributing factor. It is demonstrated that previous orthodontic treatment may have damaged the cementum layer, exposing the dentin and stimulating the clastic cells to resorb the dentin [12, 14, 23]. Two different studies found that orthodontic treatment is the most common predisposing factor, with (21.2%) and (45.7%), respectively [7, 15]. Furthermore, due to the location of the orthodontic bands, mandibular molars were shown to be one of the most affected teeth, following the maxillary incisors and canines [7, 27]. On the other side, Jeng et al. found that orofacial trauma (33.33%), followed by periodontal therapy (26.98%), and orthodontic treatment (15.87%) are the most common predisposing factors for ECR [28]. It has also been mentioned that stress may be involved with root resorption since it induces the production of cytokines such as interleukin 1 (IL- 1) and tumor necrosis factor α (TNF-α) [29, 30]. These pro-inflammatory cytokines are responsible for modulating the cellular mechanism of dental root resorption [29, 31]. Therefore, stress can also be considered as a contributing factor in our case.
In this case, the ECR was demonstrated clinically as a sudden appearance of pink discoloration in the mesio-lingual cusp of the mandibular first molar with normal pulp response. Once the ECR is diagnosed, the treatment should be conducted immediately, and it can be either conservative or surgical, according to the Heithersay class [9, 12]. The success rate for teeth observed with class 1 and 2 lesions was 100% based on an examination of 101 damaged teeth in 94 patients with a minimum of three years of follow-up [7]. The success rate noted with class 3 and 4 lesions was 78% and 2%, respectively [7]. The present case is classified as Heithersay class 2. Due to normal pulp response, periodontal status, and healthy pulp with pinpoint exposure, the treatment involved debridement of the granulation tissue, direct pulp capping using MTA, followed by Glass Ionomer and composite restoration of the cavity, and the final restoration will be indirect restoration (ceramic onlay). One case study followed the treatment progression of a class 3 ECR for 12 months after direct pulp capping and showed a successful conservative treatment outcome [32]. The stated case study reported using CEM (calcium-enriched mixture cement) for the direct pulp capping [32]. However, in our case, MTA was the chosen material as it has superior properties such as great structural integrity, biocompatibility, high sealing efficiency even in the presence of moisture, and bactericidal effect [33-38]. When comparing MTA and CEM, both show great results when used as direct pulp capping materials [39, 40].
Microscopically, the histological features of ECR with the development stages of the lesion [11, 24]. The presence of fibrovascular granulation tissue with free inflammatory cells in the resorption cavity, as in our case, usually indicates that the lesion is in its early stages [9, 14]. However, the lesion has progressed to a late stage when ectopic bone-like calcifications form [14].
CONCLUSION
ECR is an aggressive and invasive lesion that acts silently, requiring early diagnosis and treatment for a successful outcome. The lesion size, location, and accessibility are the main factors affecting the successful treatment of ECR. The two-dimensional radiograph was a helpful tool for detecting the lesion. However, CBCT was an essential tool in the diagnosis, classification, and treatment planning of ECR. Notably, using MTA as a direct pulp capping material is a crucial step in the successful treatment of ECR due to its superior properties compared to other materials.
LIST OF ABBREVIATIONS
| ECR | = External Cervical Resorption |
| CBCT | = Cone Beam Computed Tomography |
| CEJ | = Cementoenamel Junction |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
Not applicable.
CONSENT FOR PUBLICATION
Informed written consent was obtained for using patient photographs and data obtained.
STANDARDS OF REPORTING
CARE guidelines and methodology were followed.
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available within the article.
FUNDING
None Declared.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


