All published articles of this journal are available on ScienceDirect.
Effect of Seashell and Eggshell Nanoparticles on Tooth Color Changes after Bleaching Using CIE L*C*H Color Space
Abstract
Background
Demineralization of dental structures is one of the post-bleaching effects. Remineralizing agents are recommended to overcome this problem. Synthesis of biomaterials from natural sources rich in calcium remains a viable and more economical option. Post-remineralization discoloration caused by such materials could disappoint patients who prefer whiter teeth; therefore, the color changes caused by such materials should be a concern during selection of the remineralizing protocol after bleaching.
Aim
This study aimed to evaluate the effect of seashell and eggshell nanoparticles on tooth color changes after bleaching using CIE L*C*H color space.
Materials and Methods
Forty natural maxillary incisors were enrolled in this study. Teeth were divided into four groups according to the applied treatment, G1: seashells paste for 2 minutes, G2: eggshells paste for 2 minutes, G3: 2% NaF gel for 1minute, and G4: received no treatment (control), then all specimens were stored in artificial saliva for 24hrs. The color of the specimens was determined according to the CIE L*C*H color space using a spectrophotometer at baseline, after bleaching, and after remineralization. Colors were compared using the ΔL*, ΔC*, and ΔH* parameters as well as the total color difference (ΔE) after bleaching and remineralization.
Results
After bleaching; there was a statistically significant difference between the experimental groups for ∆C, ∆H, and ∆E. After remineralization, there was a statistically significant difference in the mean values of ΔL, ΔC, ΔH, and ΔE between groups, the lowest mean value of (ΔE) recorded with eggshells, followed by 2% NaF gel, then seashells, while the highest mean value of ΔE was recorded with the control group.
Conclusions
After bleaching, nano-eggshells and 2% NaF maintained the post-bleaching color, indicating the recovery of the damaged enamel surface. Enamel treatment using seashells could negatively affect the effectiveness of whitening.
1. INTRODUCTION
Dental bleaching is a simple and conservative procedure for the aesthetic restoration of vital and non-vital discolored teeth [1]. Currently, many bleaching agents are commercially available with various components and concentrations, such as hydrogen and carbamide peroxide. The low pH of some bleaching agents, by-products of protein denaturation (e.g. urea), and prolonged exposure of tooth surface to the bleaching agents can cause tooth demineralization and sensitivity [2, 3].
Some remineralizing compounds, such as fluoride, calcium, bioactive glass, arginine/calcium carbonate, and nano-hydroxyapatite; have been investigated to reduce these adverse effects [4-6]. Recently, there has been interest in developing materials with bioactive potential. As the demand for environmentally friendly technology increases, synthesis of biomaterials from calcium-rich natural sources remains a viable and more economical option [7, 8].
Eggshells and Seashells are bioactive materials, a cost-effective, renewable, and rich source of calcium carbonate. Previous studies evaluated tooth enamel's successful remineralization using biowaste materials [7-9]. However; dentists are concerned about the post-treatment discoloration caused by such remineralizing agents, which could disappoint patients who prefer whiter teeth.
To objectively determine the difference between two perceived colors, a mathematical equation is used to calculate the colorimetric distance (ΔE). The colorimetric distance is defined as the value that represents the distance between the positions of two colors within the color space [10, 11].
The most frequently used color space to calculate the color of natural teeth and restorative materials has been the standard established by the Commission Internationale de l’Eclairage in 1976 (CIE L*a*b*) [10, 11]. Later, the Commission Internationale de l’Éclairage developed the CIE L*C*H* color space based on the previous standard. Since the new color space had a better corresponds better with the perception of the human eye, it has only recently been incorporated into the assessment of the natural color of teeth [11]. CIE L*C*H* is based on a polar coordinate system, where L* (brightness), C* chroma (color intensity or saturation), and values of H* correspond to hues or shades (perceived color) [10, 11]
Until the publication of this paper, no studies used this color space to measure the color changes caused by such biowaste remineralizing materials after bleaching. Despite the great potential of eggshells and seashells and the high bioavailability of calcium in them, there is no consensus in the literature on the effectiveness of eggshells or seashells on the color changes of enamel after bleaching, so this in vitro study aimed to evaluate the color changes by these natural remineralizing agents on enamel after bleaching using the CIE L*C* H* color space.
The study tested two null hypotheses: 1) the use of eggshells and seashells after bleaching does not affect the color of the teeth, 2) there is a similar relationship between the L*, C*, and H* parameters and ΔE.
2. MATERIALS AND METHODS
Materials and solutions used in the current study, composition or/and description, and manufacturer are described in Table 1.
2.1. Methods
2.1.1. Sample Size Calculation
According to a previous study by Malekipour et al. (2019) [12] using the G power statistical power analysis program (version 3.1.9.4) for sample size determination, a total sample size (n=40; subdivided into 10 in each group) was sufficient to detect an effect size of (F=0.711), with an actual power (1-β error) of 0.95 (95%) and a significance level (α error) 0.05 (5%).
2.1.2. Specimens' Preparation
Forty freshly and non-decayed human permanent incisors were extracted for aggressive periodontitis in the Department of Oral and Maxillo-Facial Surgery at the Faculty of Dental Medicine, Al -Azhar University for Girls, Egypt. Ethical approval of the local ethic committee of the Faculty of Dental Medicine, Al -Azhar University for Girls, following the international guiding principles was obtained (Code: REC-OP-21-10). Teeth were cleaned, polished, and then stored in 1% thymol solution in a refrigerator at 4oC until used [13].
| Materials | Composition/Description | Manufacturer |
| Eggshell nanoparticles | 17 ± 3 nm size White powder |
Faculty of nanotechnology, Cairo University, Giza, Egypt |
| Seashell nanoparticles |
21 ± 5 nm size Gray powder |
Faculty of nanotechnology, Cairo University, Giza, Egypt |
| Jonoflour | 2% NaF gel | Dental Medical, Jet |
| White smile | 40% hydrogen peroxide a chemical bleaching material |
17037, Germany |
| Artificial saliva | 9g NaCl+0.24g CaCl2+0.43g KCl + 0.2gNaHCO3 all dissolved in 1 L of water |
Prepared at AL-Azhar University, Regional center for Mycology and Biotechnology, Cairo, Egypt |
2.1.3. Baseline Color (T0) Assessment
Spectrophotometer (VITA Easy shade V, Spitaglasse3. D-79713 Bad. Sackingen, Germany) was used for color measurements. Readings from the spectrophotometer were expressed in the form of CIE L*C* H* color parameters and recorded as baseline (T0) before any treatments. To standardize the area where the color was measured, the labial surface of every tooth was coated with two layers of red lacquer (Shaima nail polish, Turkey), except for a 4×4-mm2 window at the middle third of the labial surface [12].
2.1.4. Bleaching
Bleaching gel (white smile) was applied on the exposed enamel surface (roughly a 1-mm-thick layer), then the gel was rinsed carefully and dried gently using air spray according to the manufacturer's instructions. The procedure was repeated 3 times for a total of 45 minutes.
2.1.5. Second Color (T1) Assessment
Immediately after bleaching, the color parameters of each specimen were reevaluated by the same method and recorded as (T1). The difference between different assessment values at baseline and after bleaching for each color parameters was calculated (T0-T1), and expressed as ΔL*, ΔC*, and ΔH*, then the total color difference (ΔE) after bleaching was calculated using the following equation following Peskersoy et al., (2014) [13].
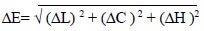
2.1.6. Sample Grouping
The forty teeth were divided into 4 main groups (n=10) according to the applied treatment (G), G1: seashell nanoparticles, G2: eggshell nanoparticles, G3: 2% NaF gel, G4; teeth were received no treatment (control).
2.1.7. Treatment Application
The specimens of groups (G1) and (G2) were treated using seashell and eggshell nanoparticles, respectively. Slurry was prepared from each powder by adding 0.3 mL of distilled water into 1.8gm of the tested powder. A volume of 0.1ml thickness of the tested pastes was measured in a disposable insulin syringe and then dispensed on the labial surface of tooth enamel and left on the surface for 2 minutes [7]. For group (G3); Samples were treated with 2% NaF gel; 0.1 mL of 2% sodium fluoride was measured in a disposable insulin syringe, then was applied on the enamel surface for 1 minute [14]. After the recommended time of application for each material, the pastes were washed with distilled water for 20 seconds, and then samples were stored in artificial saliva in individual containers labeled according to the divided groups for 24hrs.
2.1.8. Final Color (T2) Assessment
After the immersion in artificial saliva for 24 hrs, the color of each sample was reevaluated by the same method and recorded as (T2). The difference between different assessment values after bleaching and after remineralization for each color parameters was calculated (T1-T2), and expressed as ΔL*, ΔC*, and ΔH*. Then the total color difference after remineralization (ΔE) was determined using the same previous equation as described above.
2.1.9. Statistical Analysis
Statistical analysis was performed with IBM® SPSS® (Version 28.0; IBM Corporation), and Microsoft Excel 365. All quantitative data were explored for normality using the Shapiro-Wilk test. Data were non parametric. Kruskal-Wallis and Bonferroni test was used for the comparison of mean color change between different groups, while the Mann-Whitney U test was used for pairwise comparisons pre and post-remineralization in each group with each color parameter (∆H, L, C, E). Significance levels are set at (p≤0.05).
3. RESULTS
3.1. After Bleaching
Table 2 and Fig. (1) show the mean, SD, median, minimum, maximum, and results of the Kruskal-Wallis test, comparing the mean values of ∆L, ∆C, and ∆H, ∆E after bleaching between groups. There was a statistically significant difference between the experimental groups for ∆C, ∆H, and ∆E, whereas there was a non-statistically significant difference between the experimental groups for (∆L) Fig. (2).
3.2. After Remineralization
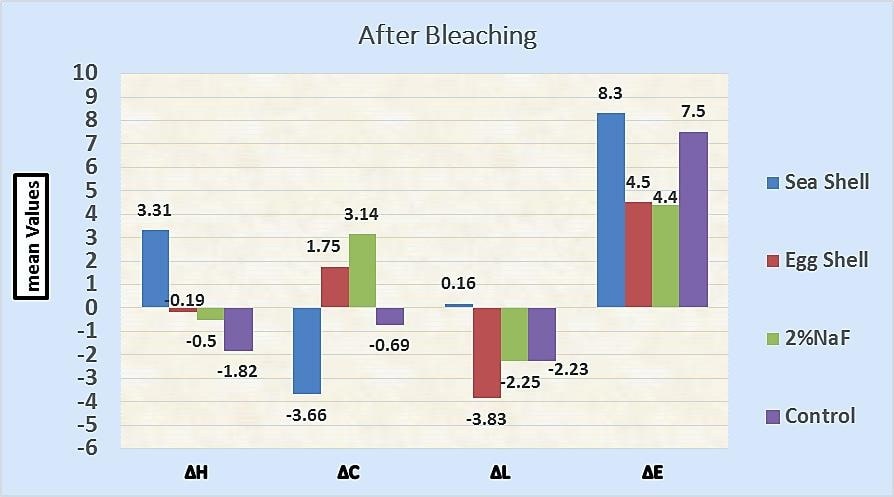
Table 3 and Fig. (2) show the mean, SD, median, minimum, maximum, and results of the Kruskal-Wallis test, comparing the mean values of ∆L, ∆C, ∆H, and ∆E after remineralization between different groups.
| Color Parameter | Groups | After Bleaching | P-value | ||||
| Mean | SD | Median | Minimum | Maximum | <0.001* | ||
| ∆H | G1 | 3.3100 a | 1.07440 | 3.7 | 1.80 | 4.30 | |
| G2 | -.1900 b | 0.89125 | -0.7 | -0.80 | 1.10 | ||
| G3 | -.0500 b | 0.00913 | -0.5 | -0.80 | 1.40 | ||
| G4 | -1.8200 b | 0.95390 | -2.9 | -3.40 | 1.20 | ||
| ∆C | G1 | -3.6600 b | 2.04189 | -3.6 | -6.20 | -1.20 | <0.001* |
| G2 | 1.7500 a | 0.49633 | 0.7 | -0.40 | 5.30 | ||
| G3 | 3.1400 a | 0.45818 | 2.9 | 0.30 | 6.30 | ||
| G4 | -0.6900 a | 0.92491 | 0 | -9.60 | 7.30 | ||
| ∆L | G1 | 0.1600 a | 0.36312 | 1.3 | -8.30 | 7.10 | 0.090 |
| G2 | -3.8300 b | 0.72600 | -3.2 | -6.70 | -1.80 | ||
| G3 | -2.2500ab | 0.78634 | -2.1 | -3.30 | -1.40 | ||
| G4 | -2.3300ab | 0.84997 | -1.4 | -6.30 | 0.40 | ||
| ∆E | G1 | 8.3320 a | 0.86300 | 8.96 | 7.33 | 9.04 | 0.005* |
| G2 | 4.5030 b | 2.89033 | 3.3 | 2.00 | 8.61 | ||
| G3 | 4.4230 b | 1.50507 | 3.61 | 3.40 | 6.60 | ||
| G4 | 7.5090 ab | 3.57390 | 7.38 | 3.22 | 11.97 | ||
Bonferroni test: Within the same comparison, means sharing the same superscript letter are not significantly different.
| Color Parameter |
Groups |
After Remineralization | P-value | ||||
| Mean | SD | Median | Minimum | Maximum | 0.047 |
||
| ∆H | G1 | -2.4300 b | 0.98385 | -3.3 | -4.10 | 0.40 | |
| G2 | -0.2700 a | 0.02095 | 0 | -1.70 | 0.60 | ||
| G3 | 0.0900 a | 0.02305 | -0.3 | -1.10 | 1.80 | ||
| G4 | 0.7900 a | 0.14655 | -0.5 | -0.60 | 3.90 | ||
| ∆C | G1 | 4.8900 a | 2.35110 | 5.7 | 1.60 | 7.10 | <0.001* |
| G2 | 1.1800 b | 0.58080 | 1 | 0.70 | 2.00 | ||
| G3 | 1.0800 ab | 0.99933 | 2.1 | -1.80 | 2.60 | ||
| G4 | -4.2900 c | 1.32451 | -3.6 | -6.20 | -3.30 | ||
| ∆L | G1 | 2.2300 a | 0.15209 | 2.5 | -1.80 | 5.90 | 0.006* |
| G2 | 0.2200 a | 0.67132 | 0.4 | -0.70 | 0.90 | ||
| G3 | 0.1500 ab | 1.84165 | 0.3 | -2.20 | 2.30 | ||
| G4 | -2.0800 b | 1.91764 | -1.3 | -4.80 | -0.40 | ||
| ∆E | G1 | 7.2160 a | 0.85223 | 7.3 | 6.12 | 8.20 | <0.001* |
| G2 | 1.7640 c | 0.66545 | 2.16 | 0.80 | 2.20 | ||
| G3 | 3.0460 b | 0.28582 | 3.04 | 2.70 | 3.40 | ||
| G4 | 5.6970 a | 1.64913 | 6.36 | 3.36 | 7.15 | ||
Bonferroni test: Within the same comparison, means sharing the same superscript letter are not significantly different.
3.2.1. (ΔL)
The Kruskal-Wallis test showed a significant difference comparing the mean values of ΔL after remineralization (P =0.006). Seashells recorded the highest mean value (2.2300), followed by eggshells (0.2200), then 2% NaF gel (0.1500), while the lowest mean value was recorded with the control group (-2.0800).
The Bonferroni test showed a statistically significant difference comparing seashell or eggshell groups to the control group. On the other hand, there was none a statistically significant difference between the tested materials.
3.2.2. (ΔC)
The Kruskal-Wallis test showed a statistically significant difference comparing the mean values of ΔC between the different groups after remineralization (P<0.001), with the highest mean value recorded with seashells (4.8900), followed by eggshells (1.1800), and then 2% NaF gel (1.0800), while the lowest mean value was recorded with the control group(-4.2900).
Bonferroni test showed no statistically significant difference between seashells or eggshells when compared to 2% NaF gel. On the other hand, there was a statistically significant difference between other treatments.
3.2.3. (ΔH)
Kruskal-Wallis test showed no statistically significant difference comparing the mean values of ΔH between different groups after remineralization (P=0.047).
3.2.4. Color Change (ΔE)
Kruskal-Wallis test showed a statistically significant difference comparing the mean values of ΔE after remineralization (P<0.001). Seashells recorded the highest mean value (7.2160), followed by the control group (5.6970), then 2% NaF gel (3.0460), while eggshells recorded the lowest mean value (1.7640).
The Bonferroni test showed a statistically significant difference comparing the mean values of ΔE for each pair of treatment, except for seashell when compared to the control group; the difference was not statistically significant.
| Color Prameters | Groups |
Mean |
P-Value |
| ∆H | G1 (pre) | 3.3100 | <0.001* |
| G1 (post) | -2.4300 | ||
| G2 (pre) | -0.1900 | 0.939 | |
| G2 (post) | -0.2700 | ||
| G3 (pre) | -0.0500 | 0.540 | |
| G3 (post) | 0.0900 | ||
| G4 (pre) | -1.8200 | 0.026* | |
| G4 (post) | 0.7900 | ||
| ∆C | G1 (pre) | -3.6600 | <0.001* |
| G1 (post) | 4.8900 | ||
| G2 (pre) | 1.7500 | 0.346 | |
| G2 (post) | 1.1800 | ||
| G3 (pre) | 3.1400 | 0.026* | |
| G3 (post) | 1.0800 | ||
| G4 (pre) | -0.6900 | 0.125 | |
| G4 (post) | -4.2900 | ||
| ∆L | G1 (pre) | 0.1600 | 0.540 |
| G1 (post) | 2.2300 | ||
| G2 (pre) | -3.8300 | <.001* | |
| G2 (post) | 0.2200 | ||
| G3 (pre) | -2.2500 | 0.026* | |
| G3 (post) | 0.1500 | ||
| G4 (pre) | -2.3300 | 0.540 | |
| G4 (post) | -2.0800 | ||
| ∆E | G1 (pre) | 8.3320 | 0.004* |
| G1 (post) | 7.2160 | ||
| G2 (pre) | 4.5030 | 0.026* | |
| G2 (post) | 1.7640 | ||
| G3 (pre) | 4.4230 | <0.001* | |
| G3 (post) | 3.0460 | ||
| G4 (pre) | 7.5090 | 0.125 | |
| G4 (post) | 5.6970 |
3.3. Post-bleaching versus Post-remineralization (Pairwise Comparison)
Table 4 shows the mean values and results of the Mann- Whitney U test for pairwise comparison between pre-and post-remineralization within each group for the color parameters ∆L, ∆C, ∆H, and total color difference ∆E.
4. DISCUSSION
Tooth bleaching is a conservative and safe method of treating discolored teeth [15]. Demineralization of dental structures is one of the important post-bleaching side effects caused by the low pH of the used materials. About two-thirds of patients who receive this treatment experience some degree of transient tooth sensitivity during and after bleaching [2, 15].The redox reactions of the bleaching material can lead to the dissolution of organic and mineral matrices to the extent that only carbon dioxide (CO2) and water remain. This compels dentists to prescribe remineralizing products [15].
Researches on different formulations of peroxide with calcium and fluoride showed that fluoride and/or calcium prevent microhardness reduction and accelerate the recovery of post-treatment microhardness to the pre-treatment state [1, 4]. Recently, researchers recommended natural biowaste products like seashell and eggshell nanoparticles; to increase the microhardness and remineralization of demineralized enamel [7-9].
Despite these benefits, using these medications after bleaching may lead to discoloration of the teeth, which may disappoint patients who desire whiter and more lustrous teeth. Therefore, this study aimed to evaluate the effect of seashell and eggshell nanoparticles on enamel color changes after bleaching.
To objectively determine the difference between the two perceived colors in this study, our work demonstrates for the first time; a mathematical equation in which the colorimetric distance (ΔE) obtained in the color space CIE L*C*H* in evaluating the tooth color changes after bleaching and mineralization. This color space is derived from another previously developed color space (CIE L*a*b*), which has been used to measure the color of teeth. However, CIE L*C*H* surpasses the previous versions since its color representation has a better correlation with the perception and interpretation of the human eye [16].
The results of this study showed a statistically significant total color alteration (ΔE) of the post-bleached treated samples, suggesting that the bleaching agent may have penetrated correctly into the enamel.
Regarding the effect of the treatments on the color change of bleached enamel, there was a statistically significant difference in the mean values of ΔE between different groups. All three remineralizing materials showed a significantly lower ΔE after remineralization compared to the post-bleaching.
The lowest mean value of ΔE recorded with eggshells, followed by 2% NaF, and the recorded ΔE was1.76 and 3.04, respectively. The human eyes can't perceive these changes as the lowest ΔE that can be differentiated by the human eyes are more than 3.3 as reported by previous study by Gawriołek et al. [17]. The mean value of 3.3 (ΔE) is reported to be esthetically acceptable, and any difference beyond this limit is highly perceptible and clinically unacceptable [17, 18].
These findings can be explained by the fact that the remineralizing substances could be penetrated to a good depth through the bleached enamel making the teeth more similar in appearance to the natural teeth.
The current findings may be in the same line with a previous study by Tay et al. who reported that, 2% NaF didn't interfere with the bleaching effect of 35% H2O2 [19]. Also, these results are consistent with the evidence provided by Armenio et al [20], who examined the effect of 1.23% fluoride gel applied for four minutes after the daily use of carbamide peroxide gel and reported that, NaF didn't disturb the whitening effect of the peroxide gel [20].
Chen et al. reported that, fluoridated bleaching agents and post-bleaching fluoride treatment did not interfere with the bleaching effect [21]. Also, Maran et al. reported that, the incorporation of NaF in at-home bleaching gel containing 10% carbamide peroxide, does not affect the color change [22].
On the other hand, the current findings are in contradiction with a previous study by Malekipour et al, have recorded the highest ΔE with 0.05%NaF mouthwash use after bleaching compared to the other solutions (ΔE= 7.29) [12]. Furthermore, a study by Kim et al. conflicts with the current findings [23]. They reported that, NaF produces a visible discoloration after bleaching (ΔE=7.51), which contradicts our results (ΔE=3.04).
To the best of our knowledge, the present study is the first investigation on the discoloration potential of both eggshells and seashells; therefore, there is no reference for comparing of the results concerning these products. In addition, Kim et al. have reported that, nano-carbonate apatite prevented the re-staining after tooth bleaching in-vitro [23], this may be consistent with eggshells results in the current study, which recorded minimal color change after bleaching (ΔE=1.76).
However, the results of seashells challenge this report, we found that seashells can cause visible discoloration in bleached teeth (ΔE=7.21). The highest color changes were recorded after using seashells (ΔE=7.21), based on the above thresholds, this qualifies as clinically perceptible by human eyes and therefore, unacceptable color differences. This probably may be attributed to many reasons; first, the gray color of the seashell powder compared to the white color of eggshell powder. Second, the pores created after bleaching may not be filled for a good depth with seashell nanoparticles like eggshells. Therefore, the first null hypothesis reported that, there would be no difference between seashells and eggshells in color changes after bleaching was rejected.
In the current study, artificial saliva may cause perceptible unacceptable color changes in bleached teeth (ΔE=5.69). This fact probably may be explained by the pellicle formed by saliva on the teeth surface may increase its stain-ability.
Detailed analysis of each of the parameters that make up the color after treatment of the bleached enamel using eggshells; showed a significant increase in the brightness (∆L), and a non-significant decrease in the color intensity (chroma) (∆C) and hue (∆H), on the other hand, it showed a significant decrease in the total color difference (∆E) after remineralization with respect to the post-bleaching effect. This fact means that (∆E) is inversely proportional to the (∆L), however it is directly proportional to other color parameters; (∆H) and (∆C), thus the second null hypothesis, which stated a similar relationship between the L*, C*, and H* color parameters and ΔE is rejected,
These findings imply that eggshells positively affect bleached enamel and can restore teeth to their normal color. This may be related to the refractive index of eggshell crystalline structure calcite (1.63), which is closest to the refractive index of the normal tooth enamel(1.65) as reported by Sabuncu et al [24], hence the difference in the refractive index between enamel and nanoparticles is reduced, the enamel returns to its translucency and looks similar to the normal teeth.
Furthermore, seashells recorded the highest value for brightness (∆L) and color intensity (∆C) (chroma), implying that they could reversibly influence and mask the bleached color. This could be explained by three reasons; first; the fact that the micro-porosities of the bleached enamel, which is filled with seashell nanoparticles, may have a refractive index far away from that of enamel, thus the difference in the refractive indices between the porosities and enamel is increased
Second; related to the volume of minerals, seashell nanoparticles may not fill the full depth of the bleached enamel by restoring the surface rather than the body of the enamel, thus increasing the optical reflectivity as most of the detected signals come from lesions, not fully remineralized. Third, related to the crystalline structure of the calcium carbonate in seashells which is (aragonite), these are responsible for the iridescent colors of the shells, as reported by a previous study by Liu et al. [25].
CONCLUSIONS
After bleaching, the nano-eggshells and sodium fluoride gel maintained the post-bleaching color, indicating that the damaged enamel surface had recovered. Enamel treatment using seashell nanoparticles could negatively affect the effectiveness of whitening.
LIST OF ABBREVIATIONS
| ∆C | = Color intensity (chroma) |
| ∆H | = Hue |
| ∆L | = Brightness |
| ∆E | = Total color difference |
AUTHORS' CONTRIBUTIONS
Fatma Hussein is involved in all stages of the research processing, including research designing, analysis, and writing. Sahar El Marsafy acted as a research and academic supervisor to Fatma Hussein, who was involved in the designing, analysis and writing of the manuscript.
ETHICS APPROVAL AND CONSENT TO PARTI-CIPATE
Ethical approval of the local ethic committee of the Faculty of Dental Medicine, Al -Azhar University for Girls, following the international guiding principles was obtained(Code: RECOP-21-10).
HUMAN AND ANIMAL RIGHTS
No Animals were used in this research. All human research procedures were followed in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Written informed consent was obtained from all the participants.
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available within the article.
CONFLICT OF INTEREST
The author declares no conflict of interest, financial or otherwise.
FUNDING
The authors declared that this study received no financial support or funding.
ACKNOWLEDGEMENTS
Declared none.


