All published articles of this journal are available on ScienceDirect.
Complementary Effect of Electro Acupuncture on Refractory Head and Neck Myofascial Pain: A Retrospective Investigation of Patient-Reported Outcomes
Abstract
Introduction:
Acupuncture (AC) is among the complementary treatment modalities to manage chronic myofascial pain. The aim of this investigation was to assess the additive effect of AC in reducing the intensity of primary chronic myalgia/myofascial head and neck pain in patients using oral orthotic appliances (OA).
Methods:
A retrospective chart review was conducted for 103 patients diagnosed with primary chronic myalgia/myofascial pain and received OA with/without AC at Tufts University School of Dental Medicine (TUSDM). Subjective reporting of face/TMJ/neck pain was recorded at the initial visit and at short-term and long-term follow-ups using patient-reported pain/discomfort numeric rating scale (NRS).
Results:
Most subjects were females (77.7%) with the mean age of the entire study population being 53 years old. In patients with refractory response to OA, combining AC with OA showed significant improvement in NRS score compared to baseline, in TMJ pain (P=0.023), neck pain (P= 0.055), facial pain (P=0.006). The addition of AC to OA has also brought refractory pain to low levels, comparable to what OA-only respondents reported [TMJ pain (P= 0.395), neck pain (P=0.694), face pain (P=0.553)].
Conclusion:
AC may provide a complementary therapeutic modality to manage refractory cases of primary chronic myofascial pain.
1. INTRODUCTION
Temporomandibular joint disorder (TMDs) are defined by the American Academy of Orofacial Pain as a group of disorders involving the masticatory muscles, the temporomandibular joint (TMJ) and associated structures [1]. Musculoskeletal disorders affecting the neck and the temporomandibular joint, along with their supporting structures, are the second most commonly reported musculoskeletal disorders, after chronic lower back pain, and are highly associated with compromised quality of life [2]. Moreover, TMDs and neck pain disorders can affect up to 12% of the US population, with an estimated annual cost of $4 billion [2, 3]. Head and neck myalgia and myofascial pain are among the most frequently diagnosed disorders that fall under the umbrella of TMDs [4]. It can be associated with trigger points, a hyperirritable taut band, generating spontaneous or triggered pain that can be confined to the affected muscle or spread to the adjacent or remote structures [5]. The etiologies of TMDs are multifactorial and yet not fully understood. Trauma and/or parafunctional activities of the face and neck musculature, in addition to stress and incorrect posture, may perpetuate myofascial pain complaints in a genetically susceptible population [6].
Acupuncture (AC) is a traditional Chinese/Japanese medicine approach that is believed to stimulate specific points in the skin to induce analgesia [7]. Frequently, AC/dry needling may make a good complementary method to treat refractory cases of myofascial pain. It can also be used as an alternative in case of failure/contraindication of the conventional treatment modalities [8]. Pain management using AC therapy has been steadily growing over the past two decades. This growth came consequently to the National Institute of Health (NIH) consensus statement published in 1997 in support of the efficacy of AC in managing cases of pain and nausea [9]. In 2002, the National Health Interview Survey (NHIS) reported that around 2.13 million Americans had used AC, with musculoskeletal complaints being the most frequently reported conditions [10].
In the head and neck region, AC has been reported to work primarily through cranial nerves stimulation, namely, trigeminal nerve in sensory disorders and facial nerve in motor dysfunctions [11]. It may also enhance salivary secretion in patients with hyposalivation through parasympathetic nerve stimulation [12]. Furthermore, AC has shown a positive effect in reducing TMD-associated pain when targeting irritable trigger points through modulation of the pain pathway and induction of anti-inflammatory actions [7, 13].
The main aim of this study was to assess, based on patients’ reporting, the additive effect of AC in reducing the intensity of head and neck primary chronic myalgia/myofascial pain in patients with OA. The secondary aim was to assess the significance of adding AC to OA in reducing other head and neck complaints associated with primary chronic myalgia/myofascial pain such as TMJ clicking tinnitus/stuffy ear, otalgia, and headache.
2. MATERIALS AND METHODS
This is a retrospective chart review investigation of patients seen at the Craniomandibular Disorders Clinic (CMDC) at Tufts University School of Dental Medicine (TUSDM) between January 2007 and January 2018. The study was approved by Tufts Health Science Institutional Review Board (IRB # 12856) and was performed in accordance with the Declaration of Helsinki. All patients seen at the CMDC were informed that their de-identified data can be used for research purposes and were consented, in writing, prior to initiation of therapy.
2.1. Inclusion Criteria
The inclusion criteria for this investigation were as follows: (a) adult patients aged 18-85; (b) patients subjective reporting of pain/discomfort in the face, TMJ area, and/or neck using the numeric rating scale (NRS) prior to the initiation of any treatment modalities; (c) objective confirmation of primary chronic myalgia/myofascial pain based on the RDC-TMD and DC-TMD [14] through clinical and radiographic examination by an orofacial pain expert; (d) persistent pain for 3 months or more; (e) documentation of none, one, or more accompanying symptoms (i.e. TMJ sound, tinnitus/stuffy ear, otalgia, and/or headache; (f) documentation of presence or absence of concurrent pharmacotherapeutics (i.e. analgesics, opioids, muscle relaxants, and/or anxiolytics); g) documentation of presence or absence of other supportive modalities such as physical therapy (PT), home self-care (i.e. thermal packs and masticatory/neck muscles exercises), and/or trigger points/Botox injections, (h) all included patients received either an OA or a combination of OA and traditional Chinese/Japanese AC or electroacupuncture, (i) documentation of adequate number of AC treatment sessions (minimum of 5 sessions) starting at 1 month ±2 weeks after the insertion of the OA, (j) adequate documentation of short-term and long-term follow ups, including patients-reported NRS scores, in the patient’s records (detailed follow-up time-point for both groups is included in the data collection section).
2.2. Exclusion Criteria
Charts of patients who did not have confirmed primary chronic face, TMJ, and/or neck myalgia/myofascial pain were not considered in this study. Those with neuropathic orofacial pain or temporomandibular disorder related to rheumatoid/connective tissue disorders were excluded. Patients with severe cognitive or mental disabilities that compromise their ability to understand and/or fill the NRS and those with uncontrolled psychological disorders that may affect their perception of treatment outcomes, were also excluded.
2.3. Data Collection
2.3.1. Demographic Data and Baseline Variables
A search query of TUSDM billing database was conducted by the IT department using the ICD-9 and ICD-10 diagnosis codes specified for myalgia and myofascial pain (ICD-9: 729.1; ICD-10:M79.1) and matched with the CPT codes for the interventions of interest (AC with electrical stimuli: 97813+; AC without electrical stimuli: 97810+; OA: 21299, S8262, D7880). A preliminary list of both paper charts (for patients seen prior to 2015) and electronic charts (patients seen during and after 2015) was created. Patients’ charts were retrieved, reviewed, and inclusion/exclusion criteria were applied. Patients’ demographics (age and gender), medical history, and secondary orofacial pain diagnoses were collected. Information about the concurrent use of supportive treatment modalities, namely, medications (analgesics, opioids, anxiolytics, muscle relaxants), physical therapy (PT), trigger point injections, Botox injections, and/or home self-care (thermal packs and masticatory/neck muscle exercises) were also extracted.
To evaluate the additive effect of AC, patients were divided into two comparison groups: oral orthotic appliances group (OA) and combination therapy of AC and OA appliance group (AC/OA).
2.3.2. Definition of Primary and Secondary Outcomes
An 11-point pain and discomfort numeric rating scale (NRS) was used to assess the patient’s pain intensity, where 0 indicates no pain at all and 10 indicates the worst pain [15]. As part of the patient care protocol at the Craniomandibular Disorders Clinics at TUSDM, all patients are asked to fill this scale on every visit while in the waiting area prior to seeing their orofacial pain provider.
Primary outcomes for this study were defined as the change in NRS scores for a) face pain/discomfort (reflecting myalgia/myofascial pain of the masseters and/or temporalis and their surrounding/supporting structures); b) TMJ area pain/discomfort (reflecting myalgia/myofascial pain of the upper part of the masseter, lower part of the temporalis, and/or the medial and lateral pterygoid muscles and their surrounding/supporting structures); c) neck pain/discomfort (reflecting myalgia/myofascial pain of the levator scapulae, splenius capitis, the upper part of the trapezius, and/or sternocleidomastoid muscles and their surrounding/supporting structures).
Secondary outcomes were defined as the change in NRS scores for other accompanying symptoms associated with myalgia/myofascial pain including a) TMJ sound; b) otalgia; c) stuffy ear/tinnitus; d) headache.
2.3.3. Definition of Baseline and Follow-up time Points
For OA group, pain/discomfort NRS scores were reviewed and collected at three different time points (baseline: prior to the initiation of any treatment modality; short-term follow-up: 1 month ± 2 weeks after insertion of OA; long-term follow-up: 3 months ± 2 weeks after insertion of the OA). For AC/OA group, patients who were not satisfied with OA therapy, results were offered AC as a complementary therapy along with OA to obtain further pain reduction. NRS scores were collected at baseline (prior to the initiation of any treatment modality), short-term follow-up (1 month ± 2 weeks after insertion of OA with no AC initiated), and long-term follow-up (3 months ± 2 weeks after initiation of AC given that a minimal of 5 AC sessions were completed).
2.3.4. Description of AC Procedure
Two protocols were used for Japanese electro-acupuncture. Patients with face and/or TMJ area pain were treated at the local points ST-6, GB-20, GB-21, while in the supine position, in addition to the distal points LI-4, and LV-3. Patients with neck pain, with or without face and/or TMJ pain, were treated with local points SI-11, SI-12, SI-13, GB-20, GB-21, while in the prone position, in addition to the distal points SI-3, and BL-60. For both protocols, ear AC was always added to the Shen Man, Point Zero, and Heart points to induce relaxation during the procedure. The Electro-Acupuncture IC-1107® stimulation device was used for stimulation at 15-30 hertz, as tolerated, for 20 minutes at the distal points. Japanese Seirin needles, size 30 were used, with the depth of insertion ranging between 4-5 mm, depending on the thickness of the muscle [16].
2.3.5. Definition of OA
Per the Craniomandibular Disorder Clinics, protocol, the appliances were full-coverage occlusal splints with 1.5-2 mm thickness, hard clear acrylic. Patients were provided maxillary appliances if they reported severe symptoms upon waking up in the morning and were instructed to use them during sleep. Those who had intensified pain toward the end of the day were prescribed mandibular appliances for diurnal use. All appliances were adjusted at the time of insertion and at the first follow-up after insertion to ensure maximum comfort and balanced occlusion on the opposing teeth and contralateral side. All patients were instructed to wear their appliances for a minimum of 8 hours every day or night.
2.4. Data Analysis
Standard descriptive statistics included means and standard deviations as well as frequencies for the measured variables. Bivariate analyses included Mann–Whitney U, Spearman correlation, chi-square and Fisher exact test. Longitudinal analyses were carried out using repeated-measures ANOVA to test the interaction between the 2 groups and 3 time points. Changes in measures of NRS scores between 2 points of time were analyzed by the Wilcoxon signed rank test, while comparing between the 2 groups at any certain point of time was conducted by a Mann-Whitney U test. Multiple linear regression analysis was conducted to control for the effect of potential significant confounders in the bivariate analyses. The data analysis was conducted through SAS statistical software (version 9.1.3; SAS Institute, Cary, NC). All statistical analysis was considered statistically significant if p < 0.05. The raw data used for statistical analyses and to support the findings of this study have been deposited in the Tufts Box repository at https://tufts.box.com/s/e4a7pubz5gyshx5llrsfxr80yx5q7lcl.
3. RESULTS
3.1. Baseline Characteristics and Demographics
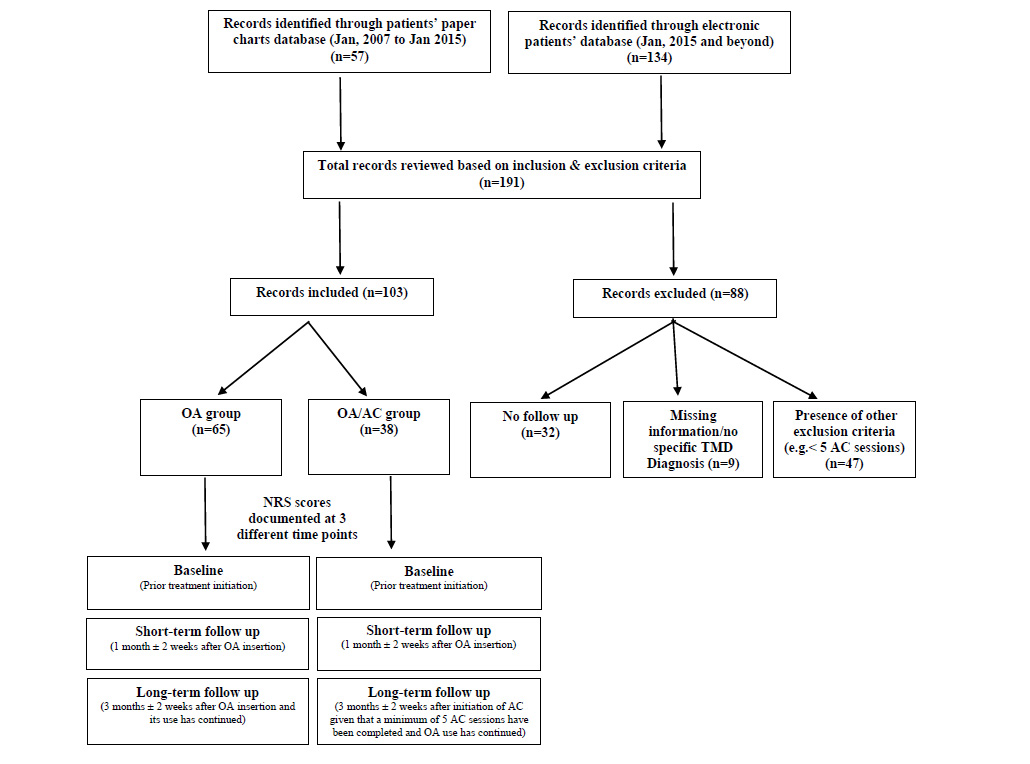
A total of 191 patients’ charts, both paper and electronic, were retrieved from TUSDM records database. Eighty-eight patients’ charts were excluded due to missing data, lacking ICD-9/ICD-10 specific TMD diagnosis, inadequate short-term or long-term follow-ups information, and/or presence of one or more of the study exclusion criteria.
A total of 103 patients’ charts were deemed eligible for further review based on the study’s inclusion and exclusion criteria. Among those, 57 received OA and 48 patients received a combination therapy of OA/AC (Fig. 1).
Out of the 103 patients, 80 (77.7%) were females. The mean age of the entire study population was 53.2 years (SD= ±16.3) with a higher mean age in the OA/AC group compared with the OA group, but with no significant statistical difference (P= 0.497) (Table 1).
| Variable | Total | †OA/AC group | ‡OA group | P value | ||||||||
| n | % | mean ± SD§ | n | % | mean ± SD | n | % | mean ± SD | ||||
| Age (years) | 103 | - | 53.2±16.3 | 38 | - | 54.6±16.6 | 65 | - | 52.3±16.1 | 0.497 | ||
| Gender |
Female Male |
80 23 |
77.7 22.3 |
- - |
28 10 |
73.7 26.3 |
- | 52 13 |
80 20 |
- | 0.472 | |
‡OA: Oral orthotic appliance therapy
§ SD: Standard Deviation
In both groups, the most common medical comorbidities were psychiatric disorders (39.8%), respiratory diseases (33.3%), and cardiovascular diseases (33%), with no significant difference in their distribution among the two groups (P=0.958, 0.527, 0.885, respectively) (Table 2). Endocrine disorders, mainly hypothyroidism, were the only medical comorbidity that had a significantly higher number in the OA/AC group compared to OA (P= 0.040), in which regression analysis was further perused (Table 2).
| Muscular Disorders Diagnosis |
Total (n=103) n (%) |
OA/AC group (n=38) n (%) |
OA group (n=65) n (%) |
P value |
| Myofascial pain (masticatory muscles) | 23 (22.3%) | 4 (10.5%) | 19(29.2%) | 0.028* |
| Myofascial pain (neck muscles) | 2 (1.9%) | 0 (0%) | 2 (3.1%) | 0.530 |
| Myofascial pain (masticatory & neck muscles) | 77(74.8%) | 33 (86.8%) | 44 (67.7%) | 0.031* |
| Myalgia | 1(0.9%) | 1(2.6%) | 0 (0%) | 0.369 |
| Pre-existing medical comorbidities | ||||
| Respiratory disorders | 34 (33.3%) | 14 (36.8%) | 20 (30.8%) | 0.527 |
| Cardiovascular disorders | 34 (33.0%) | 13 (34.3%) | 21 (32.8%) | 0.885 |
| Haematological disorders | 2 (1.9%) | 0 (0%) | 2 (3.1%) | 0.530 |
| Gastrointestinal disorders | 20 (19.4%) | 5 (13.2%) | 15 (23.1%) | 0.219 |
| CNS disorders | 15 (14.6%) | 7 (18.4%) | 8 (12.3%) | 0.396 |
| Psychiatric disorders | 41 (39.8%) | 15 (39.5%) | 26 (40.0%) | 0.958 |
| Endocrine disorders† | 17 (16.5%) | 10 (26.3%) | 7 (10.8%) | 0.040* |
| Cancer | 4 (3.9%) | 0 (0%) | 4 (6.2%) | 0.294 |
| Other TMD Disorders Diagnoses | ||||
| TMJ disorders unspecified (ICD-9:524.6 ICD-10:M26.60) | 14 (13.6%) | 6 (15.8%) | 8 (12.3%) | 0.619 |
| Arthralgia of TMJ (ICD-9: 524.62 ICD-10: M26.62) | 15 (14.6%) | 4 (10.5%) | 11 (16.9%) | 0.375 |
| Articular disc disorder of TMJ (ICD-9: 524.63 ICD-10: M26.63) | 28 (7.2%) | 6 (15.8%) | 22 (33.8%) | 0.047* |
| Other jaw pains (ICD-9: 784.92 ICD-10 R68.84) | 19(18.4%) | 1(2.6%) | 18(27.7%) | 0.001* |
| Medications | ||||
| No medications | 6(7.8%) | 2(5.3%) | 4(6.2%) | 0.852 |
| Muscle relaxants | 37(35.9%) | 16(43.2%) | 21(56.8%) | 0.688 |
| Anxiolytics | 26(25.2%) | 10(26.3%) | 16(24.6%) | 0.848 |
| Opioids | 9(8.7%) | 4(10.5%) | 5(7.7%) | 0.623 |
| Analgesics | 60(61.2%) | 22(57.9%) | 38(58.5%) | 0.955 |
| Others | 64(61.2%) | 24(63.2%) | 40(61.5%) | 0.842 |
| Other Management Aids | ||||
| None | 44(42.7%) | 17(44.7%) | 27(41.5%) | 0.752 |
| Self-care (masticatory/neck muscles exercise and thermal packs) | 23(22.3%) | 7(18.4%) | 16(24.6%) | 0.466 |
| Physical therapy (PT) | 36(35.0%) | 16(42.1%) | 20(30.8%) | 0.244 |
| Trigger points injections | 8(7.8%) | 3(7.9%) | 5(7.7%) | 0.970 |
| Botox injections | 8(7.8%) | 3(7.9%) | 5(7.7%) | 0.970 |
| Others | 5(4.9%) | 2(5.3%) | 3(4.6%) | 0.883 |
†Per charts review, all the17 subjects identified with endocrine disorders suffer from hypothyroidism. Only one of these subjects had diabetes mellitus in addition to hypothyroidism.
All patients had a primary diagnosis of chronic myalgia/myofascial pain. In both OA and OA/AC groups, 74.8% were diagnosed with myofascial pain in both masticatory and neck muscles, while 22.3% had myofascial pain in masticatory muscles only (Table 2). Secondary diagnoses were equally distributed among both comparison groups except for articular risk disorders (ICD-9: 524.6; ICD-10: M26.63), and other jaw pains (ICD-9: 784.92; ICD-10: R68.84) in which numbers were significantly higher in the OA group compared to OA/AC group (P= 0.047 and P=0.001, respectively) (Table 2).
Concurrent supportive therapies were indicated in the patients’ chart with the majority being prescribed systemic medications (94%) followed by PT (35%). No significant statistical differences in the utilization of supportive therapies were shown between the 2 groups. Table 2 provides details about the modalities of supportive therapies and their distribution among the groups.
3.2. Changes in NRS scores from Baseline, within and in between Groups
3.2.1. Primary Outcomes
3.2.1.1. TMJ Pain
At short-term follow-up, both study groups had only received OA therapy for 1 month ± 2 weeks. Within-group analysis showed a statistically significant improvement in the OA group compared to baseline NRS scores (P= 0), while no significant improvement was observed in the OA/AC group (P=0.174). At long-term follow-up (after adding a 5+ sessions of AC to the OA/AC group and continuation of OA therapy in both groups), the OA group maintained its improvement comparing with baseline scores (P=0) while the OA/AC group managed to reach a statistically significant improvement in their NRS scores (P=0.023). Interestingly, the addition of AC has brought the median difference in NRS scores (from baseline to long-term follow-up) in the OA/AC group to comparable levels to those in the OA group (P= 0.395) (Table 3).
3.2.1.2. Neck Pain
Similar to TMJ pain, short-term follow-up demonstrated a significant improvement in the median neck pain NRS scores within the OA group (P=0.07), while the OA/ACC group had no significant improvement (P=0.30), regardless the receipt of the same intervention. Long-term follow-up showed persistent improvement in the OA group (P=0) with the continuation of OA use. The addition of AC to the OA therapy had helped improve the median neck pain NRS scores in the OA/AC group to a level approximating statistical significance (P=0.055). At long-term follow-up, the addition of AC to OA therapy has approximated the median difference in neck pain score between the two comparison groups (P=0.694) (Table 3).
3.2.1.3. Face Pain
At short-term follow-up, OA therapy has significantly reduced the median NRS score in the OA group (P= 0.01) and OA/ACC group (P=0.03). Long-term follow-up showed that while the continuation of OA therapy has maintained the improvement of face pain (P=0), adding AC to the OA/AC group has further reduced the NRS scores from baseline (P=0.006). Furthermore, comparing the overall reduction in the median NRS score from baseline to long-term follow-up revealed no statistically significant difference between both OA and OA/AC groups (P=0.553) (Table 3).
3.2.2. Secondary Outcomes
3.2.2.1. TMJ Sound
Within-group comparison, short-term follow-up demonstrated significant improvement in the median NRS scores in both OA and OA/AC groups (P=0 and P=0.038, respectively). Long-term follow-up demonstrated that while a significant NRS reduction from baseline was maintained in the OA group (P=0), the OA/AC group had a further reduction in the NRS score (P=0.001). Furthermore, in-between-groups comparison showed no statistically significant difference in the median difference in NRS scores from baseline to long-term follow-up (P=0.062).
3.2.2.2. Tinnitus/stuffy Ear
Median NRS was significantly reduced in the OA group from baseline to both short-and long-term follow-ups (P= 0.02 and P=0, respectively). On the other hand, the OA/AC group encountered no statistically significant improvement neither at short-term follow-up (P=0.609) nor at long-term follow-up (i.e. after adding AC) (P=0.376).
3.2.2.3. Otalgia
Short-term follow-up revealed a significant improvement in NRS score for both OA and OA/AC groups (P=0 and P=0.031, respectively). Long-term follow-up provided a similar findings of continued improvement in the OA and OA/AC groups (P= 0 and P=0.035).
3.2.2.4. Headache
Similar to otalgia, short-term follow-up revealed a significant improvement in NRS score with OA therapy for both OA and OA/AC groups (P=0 and P=0.001, respectively). Long-term follow-up provided similar finding of continued improvement in the OA and OA/AC groups (P= 0 and P=0.002). Table 3 displays all median NRS scores for all outcomes at baseline, and short- and long-term follow-ups within and in between the groups
3.3. Controlling for Potential Confounders
While the other TMD diagnoses, articular disc disorders and other jaw pain, were significantly higher in the OA group (P=0.047 and P=0.001, respectively), the linear regression model failed to show any significant confounding effect on the changes in NRS scores for all primary outcomes at short-term and long-term follow ups. On the other hand, the endocrine disorders that were significantly higher in AC/OA group (P= 0.040), was found to be potentially confounding factor after running a regression analysis (P<0.05) (Table 4).
| Outcomes |
Baseline median (IQR†) |
Short-term follow up‡ median (IQR) |
P value1 |
Long-term follow up§ median (IQR) |
P value2 | P value3 | |
| Primary Outcomes | Pain around TMJ area OA/AC group OA group |
3.5(0.0-6.75) 5.0(2.0-7.0) |
2.0(0.0-5.75) 3.0(1.0-5.0) |
0.174 0.00* |
1.0(0.0-3.0) 2.0(1.0-3.0) |
0.023* 0.00* |
0.39 |
| Neck pain OA/AC group OA group |
3.5(0.0-7.0) 3.0(0.0-5.75) |
3.0(0.25-6.0) 1.0(0.0-4.5) |
0.301 0.007* |
2.0(0.0-4.75) 1.0(0.0-4.0) |
0.055 0.000* |
0.694 | |
| Facial pain OA/AC group OA group |
4.0(0.0-7.0) 4.0(1.0-7.0) |
4.0(0.0-5.75) 2.0(0.0-4.5) |
0.030* 0.001* |
2.0(0.0-4.0) 1.0(0.0-3.0) |
0.006* 0.000* |
0.553 | |
| Secondary Outcomes | TMJ sound OA/AC group OA group |
2.0(0.0-5.0) 2.0(0.0-5.0) |
1.0(0.0-3.75) 2.0(0.0-4.0) |
0.038* 0.000* |
0.0(0.0-3.0) 2.0(0.0-3.0) |
0.001* 0.000* |
0.062 |
| Otalgia OA/AC group OA group |
0.0(0.0-3.75) 1.0(0.0-4.0) |
0.0(0.0-4.0) 0.0(0.0-3.0) |
0.031* 0.000* |
0.0(0.0-2.0) 0.0(0.0-1.0) |
0.035* 0.000* |
0.334 | |
| Tinnitus/Stuffy ear OA/AC group OA group |
0.0(0.0-4.0) 1.0(0.0-4.5) |
0.0(0.0-5.0) 0.0(0.0-3.0) |
0.609 0.002* |
0.0(0.0-3.75) 0.0(0.0-3.0) |
0.376 0.000* |
0.236 | |
| Headache OA/AC group OA group |
4.50(1.25-7.75) 3.0(1.0-6.0) |
0(0.25-5.75) 1(0.0-4.0) |
0.001* 0.00* |
2.0(0.0-4.75) 1.0(0.0-4.0) |
0.002* 0.000* |
0.555 | |
‡ Short-term follow up: 1 month ±2 weeks after the insertion of OA.
§ Long-term follow up: 3 months ±2 weeks after insertion of the OA in the OA group; 3 months ±2 weeks after the initiation of AC given that a minimum of 5 AC sessions have been completed for the OA/AC group; Continuation of OA use during the long-term follow up period was mandatory.
P value1: for changes in median NRS from baseline to short-term follow up within each group; P value2: for changes in median NRS from baseline to long-term follow up within each group; P value3: for comparison of the changes in median NRS score from baseline to long-term follow between the OA and the OA/AC groups
*Indicates statistical significance
| - |
TMJ pain (P value) |
TMJ sound (P value) |
Face pain (P value) |
Neck pain (P value) |
Stuffy ear/tinnitus (P value) |
Ear pain (P value) |
Headache (P value) |
||||||||
| Short-term FU † | Long-term FU ‡ | Short-term FU | Long-term FU | Short-term FU | Long-term FU | Short-term FU | Long-term FU | Short-term FU | Long-term FU | Short-term FU | Long-term FU | Short-term FU | Long-term FU | ||
| Endocrine disorder | 0.018* | 0.010* | 0.001* | 0.014* | 0.003* | 0.000* | 0.003 | 0.060* | 0.456 | 0.120 | 0.083 | 0.002* | 0.456 | 0.000* | |
| Articular disc disorder | 0.674 | 0.898 | 0.175 | 0.505 | 0.653 | 0.823 | 0.738 | 0.480 | 0.370 | 0.239 | 0.925 | 0.906 | 0.034* | 0.293 | |
| Jaw pain | 0.150 | 0.183 | 0.768 | 0.899 | 0.900 | 0.030* | 0.995 | 0.426 | 0.905 | 0.983 | 0.719 | 0.666 | 0.890 | 0.110 | |
‡ Long-term FU: 3 months ±2 weeks after the initiation of AC given that a minimum of 5 AC sessions have been completed; Continuation of OA use during the long-term follow up period was mandatory.
*Indicates statistical significance.
4. DISCUSSION
The utilization of AC therapy has been increasing among the US population. Between 2002 and 2007, Zhang et al. reported that AC treatment increased from 4.2% to 6.3% of the population, representing 8.19 million and 14.01 million users in 2002 and 2007, respectively [17].
4.1. Primary Outcomes
This study demonstrated that AC may be an effective complementary modality in cases of primary chronic myofascial pain that remains refractory after OA therapy. In patients with refractory TMJ and/or neck pain, the addition of AC to OA therapy exhibited a pain/discomfort relieving effect comparable to the relieved OA respondents experienced. Patients with face pain in both groups experienced significant improvement with OA therapy (with more abundance in the OA group). Patients who improved, but were not completely satisfied with OA therapy results in OA/AC group received additional AC treatment, which increased the significance of their improvement and brought it to a level comparable to that experienced by the OA group.
Several investigations have compared the effectiveness of AC to other conventional treatment modalities in managing head and neck chronic myofascial pain. In a randomized clinical trial (RCT), Itoh and colleagues compared the effect of trigger point AC with sham AC in 13 patients diagnosed with chronic myofascial pain. After 10 weeks of treatment, they reported a significant reduction in pain intensity scores in the trigger point AC group compared to the sham group. However, no significant improvement was demonstrated in the mandibular range of motion [18]. Moreover, List et al. conducted an RCT for 110 patients diagnosed with TMDs of primarily muscular origin. The study compared the effect of AC (6 sessions) with OA (7-8 weeks) in treating TMD symptoms. While a reduction in pain intensity was witnessed in both treatment groups, AC was significantly superior to OA therapy [19].
In the contrary, Johansson and colleagues found that both AC and OA therapy significantly lowered the subjective symptoms and clinical signs of muscle-originated TMDs, with no significant difference between the two groups at 3-month follow-up. They concluded that AC may be used as an alternative method for patients who lack compliance with occlusal splint therapy [20].
Only a few studies evaluated the complementary role of AC in managing head and neck myofascial pain that has been refractory to conventional therapy. Goddard, in his retrospective study of 25 patients, generated the theory that AC may play a significant complementary role in managing a patient with chronic orofacial pain. The study called for future investigations in which a bigger number of study participants, comparison with placebo, and assessment of long-term outcomes should be considered [21]. Ferreira et al., conducted an RCT of 20 patients diagnosed with TMD in which the control group received OA therapy versus the test group that received a combination of OA and AC. In concordance with the findings from our study, they concluded that AC exhibits a significant synergetic effect with OA therapy that may expedite symptoms and relieve them. The longevity of the effect could not be concluded from the study due to the lack of long-term follow‐up [22]. List et al. retrospectively investigated the effectiveness of OA therapy versus AC in managing myogenic TMDs in a cohort of 80 patients. It was concluded that both modalities were comparably effective at both short-term and long-term follow-ups (i.e. 6- and 12-month follow-ups). However, the findings from their study failed to show a significant effect of AC/OA combination therapy in refractory cases, which contradicts the results obtained from our study [23].
4.2. Secondary Outcomes
TMJ sound was significantly improved in both groups at short-and long-term follow-ups. The further improvement in TMJ sound in OA/AC group after the addition of AC treatment might have played an indirect role by reducing the tightness of masticatory muscles related to TMJ. However, this result should be taken with skepticism as this improvement might be related to the prolonged use of OA, which was proven to be significantly effective from the very beginning.
Additional AC therapy did not improve refractory tinnitus/stuffy ear symptoms in OA/AC group in comparison to the patients who received OA alone. Strom et al. demonstrated, in their clinical study, that combining AC and intraoral splints had a favorable effect on tinnitus, which contradicts the findings obtained in this investigation [24].
Otalgia and headache were significantly reduced in both OA and OA/AC groups at short-term follow-up (with OA therapy only). Improvement also continued at long-term follow-up which may question any additive effect of AC in managing both symptoms. Kostrzewa-Janicka et al., concluded similar results in their clinical study of 43 patients. where OA therapy was effective in managing patients with myofascial pain and tension-type headache [25]. Xue et al., reported in their crossover RCT that electroacupuncture was effective in managing tension-type headache [26]. On contrary to our results, an RCT by Saha et al., found that combining OA with usual care in patients with chronic headache and comorbid TMD yielded no superior effectiveness in treating headache compared to usual care alone [27].
4.3. Confounding Effects on Outcomes
Interestingly, the study demonstrated that the higher number of subjects with hypothyroidism in OA/AC group has created a confounding effect that may have hindered the effectiveness of OA and AC therapies on all outcome measures. Hypothyroidism is associated with persistent chronic myalgia due to reduction of thyroid hormone (T3) levels and elevation of thyroid-stimulating hormone (TSH) [28]. Moreover, feedback elevation of reverse T3 (rT3) along with an increase in TSH level in patients with hypothyroidism may play an important role in both chronic myofascial pain and fibromyalgia development [29]. This may have contributed to the lack of response to OA alone in the OA/AC group at short-term follow-up. The significant improvement of these refractory cases after adding AC therapy may not only be related to the AC analgesic effect but rather correlated to an underlying mechanism to manage thyroid hormone imbalance [30].
4.4. Study Strengths and Limitations
In this retrospective study, the efficacy of AC, as an adjunctive treatment modality, comes in support of findings from several clinical investigations where evidence for efficacy and safety of electro-acupuncture was proven. Chen et al. demonstrated the efficacy and safety of long-term transcutaneous electroacupuncture in managing delayed gastric emptying after distal gastrectomy [31]. In a randomized clinical trial, Cai et. al. provided evidence for the efficacy and safety of electroacupuncture for post-stroke depression [32]. Moreover, an open-label study, by Wang et. al., proved the clinical efficacy and safety of electro-acupuncture for diabetic peripheral neuropathy [33].
The retrospective nature of this study has limited the ability to obtain accurate and highly standardized information. Thus, readers should bear in mind that the continuation of improvements in some of the outcomes could be partially attributed to concurrent interventions aiming to manage other patient-reported complaints. Moreover, missing data has led to the exclusion of a significant number of patients, especially those who received AC only. This hindered investigation of the effect of AC in the third comparison arm. In addition, the diagnosis of chronic myalgia/myofascial pain was based on the combined RDC-TMD and DC-TMD diagnostic criteria, which may have compromised the homogeneity of the study population. Information regarding intra-oral findings, such as tooth wear, and reporting of parafunctional activities were lacking in this study. Such information should be collected and precisely documented during dental examination in future studies. Furthermore, undetailed documentation regarding the specifications of OA therapies and the duration of their daily use may have masked some of the modifying effects that would skew the outcomes of the interventions.
CONCLUSION AND FUTURE DIRECTION
AC, as a complementary modality, may be helpful in managing patients with primary chronic myofascial pain that remains refractory to OA therapy. Due to the retrospective nature of this study, proof of effectiveness via cause-and-effect relationship may not be claimed. However, the positive results in this study can draw further attention to the correlational effectiveness of this approach. Conducting randomized clinical trials to prove the effectiveness of AC therapy might be essential. Utilizing a validated pain inventory such as Melzack-McGill pain questionnaire [34] and including objective outcome measures (i.e., improvement in range of motion, changes in TMJ sound, etc..) is something to consider in future investigations to obtain and more reliable multidimensional pictures.
LIST OF ABBREVIATIONS
| NRS | = Numeric Rating Scale |
| TMDs | = Temporomandibular joint |
| PT | = Physical Theraphy |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Tufts Health Science Institutional Review Board (IRB # 12856).
HUMAN AND ANIMAL RIGHTS
No animals were used for studies that are the bases of this research. The reported experiments in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
All patients seen at the CMDC were informed that their deidentified data can be used for research purposes and were concented, in writing, prior to initiation of therapy.
STANDARDS OF REPORTING
STROBE guidelines were followed.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article is available in the online Tufts Box shared drive at https://tufts.box.com/s/ss28mivu6kb679nr6zvcduaeymxiewx9.
FUNDING
The authors received no financial support for the research, authorship, and/or publication of this article.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest regarding the publication of this paper.
ACKNOWLEDGEMENTS
Declared None.


