All published articles of this journal are available on ScienceDirect.
Advances in the Management of Dentin Hypersensitivity: An Updated Review
Abstract
Background:
Objective: The objective of this narrative review is to present an overview of dentin hypersensitivity and of the prevalence, etiology, mechanism, diagnosis, and clinical management.
Methods:
Available articles (research, reviews, commentary, views, or editorial) on hypersensitivity were searched and reviewed from January 1990 till March 2021 in Pubmed, Scopus, Google Scholar, and Science Direct. Relevant studies in English were included and critically analyzed in this article.
Results:
Dentin hypersensitivity causes severe pain. The most effective and least invasive remedy is using desensitizing toothpaste. In addition, based on the dentin hypersensitivity severity, management can be done professionally in-office and through self-applied at-home treatments.
Conclusion:
Although dentin hypersensitivity causes severe pain, it can be treated using a proper remedy. Correct diagnosis helps in the proper management of dentine hypersensitivity.
Management of dentinal hypersensitivity should be done with more conservative strategies first, followed by irreversible dental interventions.
1. INTRODUCTION
Dentin hypersensitivity is a frequently encountered pain that is associated with numerous factors [1-3]. Its prevalence ranges from 3 to 98% [4-6]. Dentin hypersensitivity is a persistent clinical problem that poses a significant challenge for clinicians and affects patients’ quality of life [7]. Patients often inquire about dentin hypersensitivity in routine dental examinations. Most commonly, it occurs while drinking cold beverages, breathing, and/or eating hot foods. Its diagnosis may sometimes be challenging and can be done by excluding other dental diseases or defects, such as dental caries, broken tooth, or periodontal disease [8, 9]. Accurate diagnosis is important for its appropriate treatment.
There has been a considerable advancement and development in the management of dentine hypersensitivity. The objective of this narrative review is to present an overview on dentin hypersensitivity and on its prevalence, etiology, mechanism, diagnosis, and clinical management. Available articles on hypersensitivity were searched and reviewed from January 1990 till March 2021 in Pubmed, Scopus, Google Scholar, and Science Direct databases. Relevant studies in English were included and critically analyzed in this article.
2. PREVALENCE OF DENTIN HYPERSENSITIVITY
Dentin hypersensitivity is distributed worldwide, and its prevalence ranges from 3 to 98% [4, 10]. A new prevalence study could expect to find a prevalence of dentin hypersensitivity anywhere from 4.8% to 62.3% [7]. A study found dentinal sensitivity to be present in different populations (U.K. and Korean-based populations) and that more females complained of sensitivity than males in both the groups [10]. In a study conducted in the United States, 1 in 8 participants from general practices had dentin hypersensitivity, which was a chronic condition causing intermittent, low-level pain [6]. Patients with hypersensitivity were more likely to be younger and belonging to female sex [6].
Generally, it is observed that people of age group 30-50 years, especially females, are more affected by dentin hypersensitivity [1, 10]. It affects all teeth but is more common in canines and premolars. Most patients present dentin hypersensitivity following scaling and/or root planing. Some people also present sensitivity even when teeth are dried with air spray or slightly scratched with a probe or explorer. Cavity preparation for restoration or crown preparation involving vital teeth from the crown/bridge also causes teeth sensitivity till restoration is complete [11].
3. ETIOLOGY AND MECHANISM OF DENTIN HYPERSENSITIVITY
Dentin hypersensitivity presents with various multiple etiologies [1]. It can be caused from scaling, gingival attachment loss, and from periodontal surgery [11]. Generally, the root sensitivity occurs for 1 to 3 weeks following scaling and periodontal therapy, after which it slowly decreases. Loss of enamel causing dentin to be exposed due to teeth wear (attrition), erosion, parafunctional habits (bruxism) or other habits can also contribute to dentin hypersensitivity. One study attributed dentin hypersensitivity to gingival recession as a predisposing factor and cold stimuli as the precipitating factor [5]. The hypersensitivity is a highly prevalent cause of gingival recession and tooth whitening [6, 12]. Acids from foods and drinks, and gastrointestinal reflux can result in erosion of dentin, causing dentin tubules to be exposed.
Dentin contains dentin tubules, which are canals that radiate outward from the pulp cavity in the dentine [13]. Each dentin tubule contains various Tomes fibers and odontoblasts that are connected with the pulp. The dentin tubules have two kinds of nerve fibers, unmyelinated (C-fibers) and myelinated (A-fibers) [14-16]. The A-fibers are accountable for the sensation of dentin hypersensitivity, which is perceived as pain.
According to hydrodynamic theory, there is an outward flow of fluid along the dentin tubules [14]. They hypothesized that stimuli applied to the dentin increase fluid flow, which triggers the pulpal nerves [9]. Furthermore, Brännström et al. [17, 18] explained that the physical stimuli to dentin result from the outward flow of the dentinal fluids by means of capillary action, which results in the stimulation of the A-type nerve fibers, causing dentinal sensitivity.
4. CLINICAL SIGNS OF HYPERSENSITIVITY
Exposed dentinal surface and root surface are major predisposing factors to dentin root hypersensitivity. Although it can occur at any age, the adults >60 years old are at risk of gingival recession [1]. The clinical examination may present gingival recession, inadequate attached gingiva, exposed root surface, cervical abrasion, or bony dehiscence, as shown in Fig. (1).
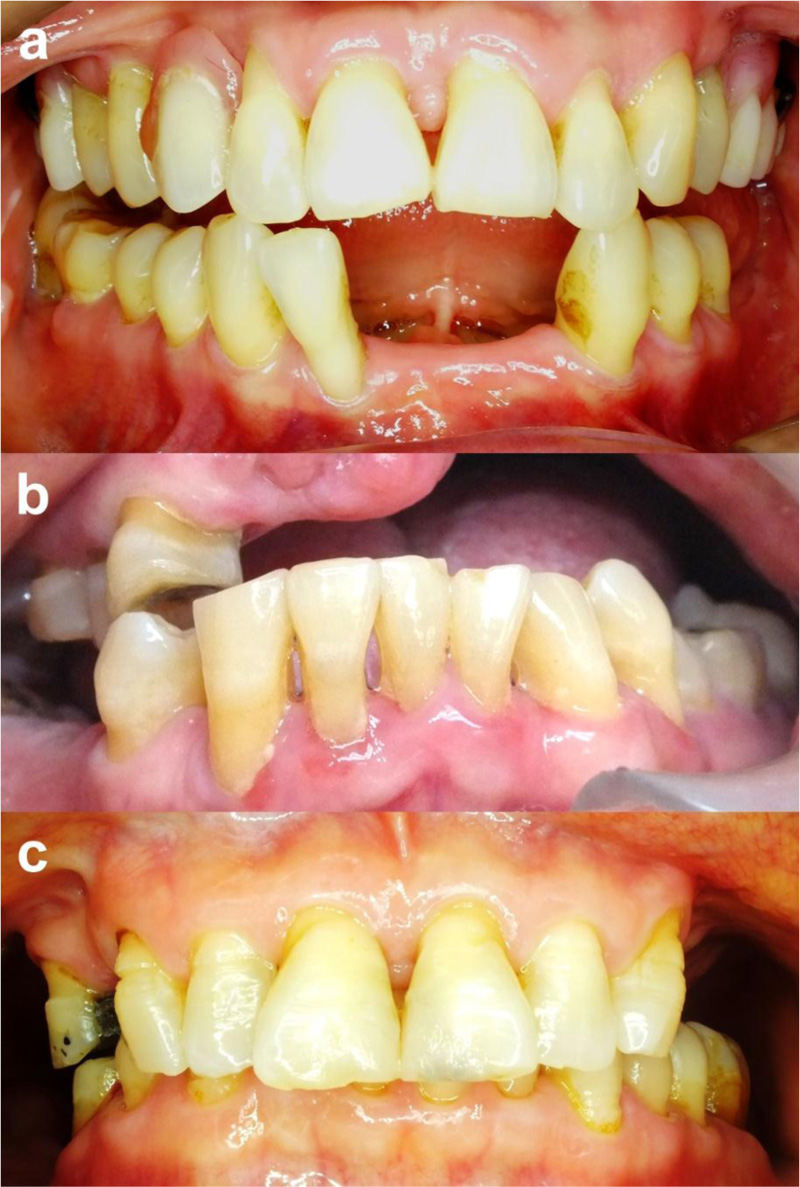
5. SCREENING AND DIAGNOSIS OF DENTIN HYPERSENSITIVITY
First, the dentin sensitivity pain should be distinguished whether it is for a short duration or a long duration. A severe night pain that lingers or wakes a person from sleep is mostly the pulpal pain. Hence, the clinician should know the etiology of dentin hypersensitivity. There are no universally accepted guidelines for differential diagnosis as well as the selection of reliable treatment modalities for this condition [19]. Generally, the dentin pain triggers when the air is blown at the tooth surface due to exposed dentin.
6. PREVENTION AND TREATMENT OF DENTIN HYPERSENSITIVITY
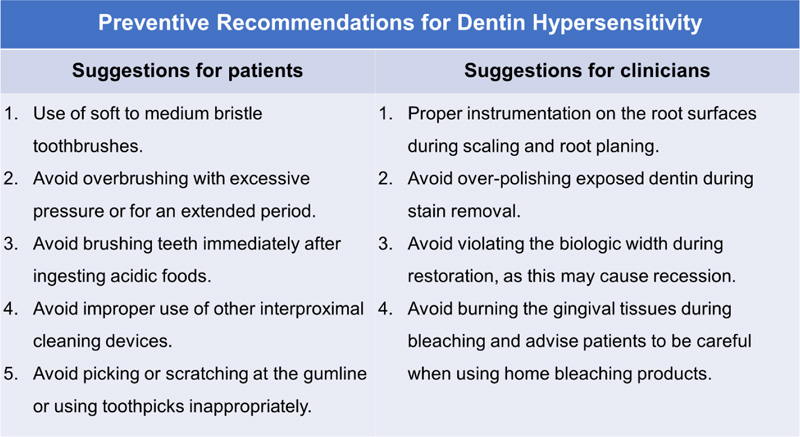
Once the dentin hypersensitivity is diagnosed, a treatment plan can be developed accordingly [20-22]. In addition, the etiology must be established. A tooth or teeth that are susceptible to dentin hypersensitivity should be located. Proper brushing techniques should be taught to prevent trauma to dentin. To control the pain during scaling and root planing, the use of topical and local anesthetic agents should be considered to relieve dentin hypersensitivity [1, 23]. The recommendations for the prevention of dentin hypersensitivity are shown in Fig. (2). Management of dentinal hypersensitivity should be done with more conservative strategies first, followed by irreversible dental interventions [3].
The basic principles for the prevention of dentin sensitivity are blocking the dentinal tubules or chemically blocking the pulpal nerve [24]. Hence, two important agents used for dentin hypersensitivity are those that (a) occlude dentinal tubules and (b) interfere with the transmission of neural impulses. The treatments for dentin sensitivity include professional in-office, at-home or over-the-counter treatments. Mild and localized dentin hypersensitivity can be treated in the office. The generalized dentin hypersensitivity with recession on multiple teeth can be treated at home. These are explained in the following sections.
6.1. Professional in-Office Treatments
In-office treatment is given to occlude and seal the dentin tubules. Generally, it is done for root sensitivity. Desensitizing resin can be applied over the cervical and/or root surface per visit.
6.2. Professional at-Home Treatments
A professional at-home treatment is launched by 3M/ESPE, which uses calcium sodium phosphosilicate bioactive glass for the treatment of sensitivity. Amorphous calcium phosphate and casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) are effective for sensitivity after brushing the teeth over 12 weeks [25-28]. ACP and CPP-ACP have also been incorporated into in-office bleaching gels [29]. Similarly, Photobiomodulation (PBM) with low-level laser therapy is effective for postbleaching sensitivity during three weeks of office bleaching treatment without compromising the quality of the treatment [30].
6.3. Self-Applied Over-the-Counter Treatments
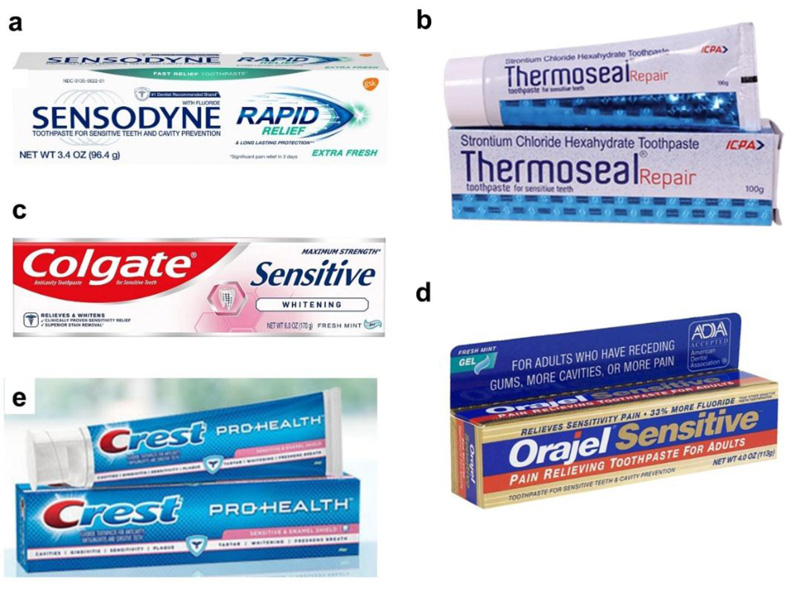
Over-the-counter (OTC) treatments include self-medication involving desensitizing toothpastes to relieve dentin sensitivity. Toothpaste with ingredients as potassium nitrate or stannous fluoride helps prevent tooth sensitivity. The mechanism of action involves the dissemination of the potassium ions through the tubules to the A-fibers and decreasing the excitability of these nerves. Some examples of such products are Sensodyne®, RA Thermoseal, Colgate-Palmolive, Colgate® Sensitive, Crest® Sensitivity, Colgate® Sensitive Enamel Protect™, Triguard, and Oral Care Gel. The active ingredients of Sensodyne® are potassium nitrate or stannous fluoride, of RA Thermoseal are potassium nitrate 5% and sodium monofluorophosphate 0.7, of Colgate® Sensitive are potassium nitrate or stannous fluoride, and of Colgate-Palmolive are calcium carbonate, sodium monofluorophosphate, zinc oxide, zinc citrate, and sodium bicarbonate.
These ingredients provide relief from dentinal pain by forming a neurosensory block. Fluoride reduces the demineralization of enamel and dentin by decreasing the acid production of bacterial plaque and the solubility of apatite crystals. It readily becomes incorporated as a fluoroapatite layer. Using it twice a day for up to 2 weeks provides effective results. Various desensitizing toothpastes and gels are shown in Fig. (3).
6.4. Recent Approaches
Various biomaterials and techniques are being developed, which can be used in the management of dentin sensitivity [22, 31-33]. Recently, arginine, a natural product, and calcium carbonate are effective for dentin sensitivity. A toothpaste containing 8% arginine, 1450 ppm fluoride, and calcium carbonate (sodium monofluorophosphate) is effective in reducing dentin sensitivity compared to 2% potassium ions toothpaste [34]. The arginine containing toothpaste seals dentin tubules with a plug that contains arginine, calcium carbonate, and sodium phosphate.
In addition, a toothpaste containing calcium sodium phosphosilicate shows improvement in salivary biochemical characteristics over 12 weeks and is effective in reducing hypersensitivity [25]. Similarly, Sensistat® containing arginine is effective in relieving hypersensitivity by occluding dentinal tubules [35]. They are positively charged and bind to the dentin surface, which is negatively charged, and attract calcium from the saliva to block the dentinal tubules. Following immediate application, this paste results in a reduction in sensitivity by 71.7% as measured by air-blast and 84.2% as measured by the “scratch” test.
Resin-based materials are also reported to reduce dentin hypersensitivity effectively. 5% sodium fluoride varnish is effective when painted over exposed root surfaces [36, 37]. A solution of glutaraldehyde and hydroxyethylmethacrylate (HEMA) has also been shown to be effective for up to 9 months due to the occlusion of tubule by glutaraldehyde [38, 39]. Oxalates are also effective causing the formation of oxalate precipitation, which occludes the open dentinal tubules.
Other modern treatments include thin coatings, adhesive resins, topically applied agents, and lasers [21, 40-42]. Iontophoresis uses a low galvanic current to exchange ions and precipitate calcium from fluoride gels, which results in the occlusion of open tubules. The treatment should not only focus on relieving pain but should also focus on curing etiology and dental problems, such as cervical abrasion, gingival recession, etc [4]. Follow-up and maintenance of hygiene are important for the prevention of dentin sensitivity.
CONCLUSION
Proper diagnosis of dentin hypersensitivity is important for the management of dentin sensitivity. Various treatments for dentin sensitivity include professional in-office, at-home, and over-the-counter treatments. Desensitizing toothpaste is the least invasive and the most cost-effective treatment. The treatment for dentin hypersensitivity should not only focus on relieving pain but should also focus on curing the etiology and dental problems. Management of dentinal hypersensitivity should be done with more conservative strategies first, followed by irreversible dental interventions.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


