All published articles of this journal are available on ScienceDirect.
Pharmacological Approach to Atypical Odontalgia Patients: A Systematic Review of Case Reports
Abstract
Background:
Atypical odontalgia (AO) is a poorly understood condition of orofacial pain and is often misdiagnosed. The pharmacological approach varies from case to case. This may be due to the differences in etiology, clinical symptoms, as well as comorbidities in each patient.
Objective:
This systematic review aimed at identifying and comprehending the adequate pharmacological approaches in AO management.
Methods:
In this systematic review, case reports that used pharmacological approach(es) as part of the AO management in the last 10 years (2010 to 2020) were reviewed using the PRISMA analysis guidelines. Initial screening was performed using keywords and inclusion criteria in several databases. Subsequently, further screening was carried out by checking for any duplication, followed by an assessment of the title and abstract and the entire content of the case report. All three authors were independently involved in studies selection based on the inclusion criteria, data extraction, and bias assessment.
Results:
Five hundred and ninety-five articles were identified from the initial search. The final result consisted of eleven case reports reviewed in this study. The use of antidepressants in AO management was reported in eight cases, anticonvulsants in five cases, antipsychotic in two cases, opioid analgesics in two cases, and topical analgesics in two cases.
Conclusion:
Based on this systematic review, various pharmacological agents showed adequate results as AO management. Antidepressants seemed to be the most effective ones (PROSPERO registration ID: CRD42021245918).
1. INTRODUCTION
Atypical odontalgia (AO) is a poorly understood condition of orofacial pain and is often misdiagnosed [1]. Patients with AO often experience continuous pain in the teeth, gingiva, or tooth socket. It often involves other areas of the face without showing any clear pathology on the teeth and periodontal tissues on clinical or radiographs examination [1, 2]. The characteristics of pain in AO can be dull, burning, sharp, or throbbing pain. Pain is non-paroxysmal and mainly persists throughout the day and lasts for more than six months or years [1, 3]. There is no representative data regarding the prevalence of AO [4]. Available epidemiological data were only obtained from the University of Southern California Orofacial Pain and Oral Medicine Center, where AO was found in 2.1% of a total of 3000 people [1, 4]. Other studies have also reported that AO occurs in 3-6% of patients undergoing endodontic treatment [2]. The cause of AO is still unclear. However, several theories about the causes of AO have been proposed by several researchers, describing neuropathic, psychogenic, vascular, and/or idiopathic factors [3, 5]. Recent studies have concluded that neuropathic factors are the most widely accepted factor as a cause of AO. The hypothesis is that most AO cases are usually preceded by trauma to the teeth [6].
As a part of chronic orofacial pain, AO is also known to have a negative impact on the quality of life and daily activities of the patient [7, 8]. This is due to pain and dysfunction complaints during oral activities, such as chewing, talking, opening or closing the jaw, and brushing teeth [9]. In addition, patients with chronic orofacial pain often experience comorbidities in psychosocial effects and behavioral disorders, such as depression, anxiety, sleep disturbances, adverse dietary changes, social isolation, or other chronic pain [10-12]. Therefore, the management of AO must be adequate. Unfortunately, dentists often misperceive the pain that occurs in patients with AO and consider the pain to be odontogenic. The patients often receive unindicated treatments, which do not reduce or eliminate the pain [1, 2, 9, 13].
Since neuropathic factor is the most widely accepted etiology, several types of neuropathic pain drugs can be used to treat AO [5]. These drugs include antidepressants, anticonvulsants, topical therapies, such as topical lidocaine and topical capsaicin, opioid analgesics, local anesthetics, and non-steroidal anti-inflammatory drugs (NSAIDs) [2, 5, 6, 14-17]. However, a pharmacological approach utilized as treatment of AO is individual. This may be due to the differences in etiology, clinical symptoms, and comorbidities experienced by different patients [2]. A literature study conducted by Melis et al. in 2003 on the pharmacological approach to AO cases from 1984 to 1998 stated that amitriptyline is a drug often used to treat AO. However, no similar study has explained any novelty in the drugs used in AO management until now [3]. Based on the abovementioned considerations, the authors consider the need for a systematic review to identify and understand various adequate pharmacological approaches in AO management.
2. MATERIALS AND METHODS
This systematic review was conducted by three authors independently following the PRISMA analysis guidelines. Three authors [YS, FP, TM] formulated the study protocol, set the inclusion and exclusion criteria, and performed data extraction. Two authors [YS and TM] independently performed the critical appraisal process and data syntheses. The search strategy was formulated by combining several keywords with the Boolean operators (OR / AND) method. The keywords used were (“atypical odontalgia” OR “persistent dentoalveolar pain” OR “persistent idiopathic dentoalveolar pain”) AND (“treatment” OR “drugs of choice”). The literature search was performed using databases, such as PubMed, Scopus, ScienceDirect, CINAHL, and Dentistry and Oral Sciences, between 2010 and 2020. The search results were selected by title and abstract, followed by full-text content based on the selection criteria. This process was conducted from January until March 2021.
2.1. Selection Criteria
The literature reviewed in this study is a full-text case report in English that utilized pharmacological approach as part of AO management with exclusion criteria in the form of case reports that do not report the patient's recovery, or the patient does not complete the treatment plans so that the effectiveness of the pharmacological agents given as AO treatment cannot be concluded.
2.2. Assessment of Quality and Risk of Bias
Case reports reviewed in this study were critically evaluated using The Joanna Briggs Institute (JBI) critical appraisal tools developed by JBI. This instrument consists of eight questions related to the current clinical condition and patient’s history, assessment method, interventions or treatment procedure, side effects, and the post-intervention clinical condition [18]. The overall quality of the case reports included divided into three categories, including (1) Low risk of bias (fulfilled > 75% of the assessment result) (2) Moderate risk of bias (fulfilled 50% - 74% of the assessment result) (3) High risk of bias (fulfilled < 49% of the assessment results) [19].
2.3. Data Extraction
The data taken from each case report includes the author, patient’s age, sex, chief complaints, precipitating factors, concomitant condition, differential diagnosis, treatments (pharmacological agent), and treatment outcomes. The results of the data extraction are presented in tabular form.
3. RESULTS
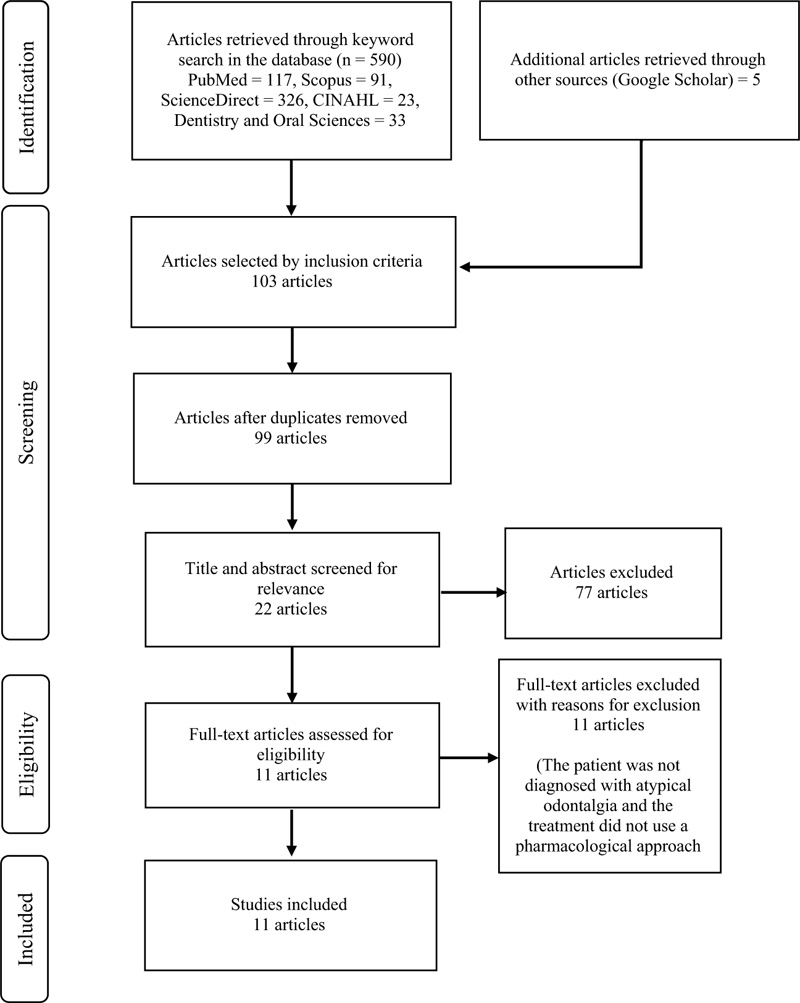
Five hundred ninety-five articles were identified from the initial search, followed by another screening based on the inclusion criteria, where 103 articles were obtained. The subsequent screening was carried out by checking for duplication, where four duplicates identified in five databases were identified, and therefore, omitted from the list. Consequently, 99 articles were obtained. The final screening was carried out based on the suitability of the entire content, where 11 articles were found suitable and included in the current systematic review. The PRISMA flow chart of the results of this search is shown in Fig. (1).
3.1. Critical Appraisal
The critical evaluation of included case reports is presented in Table 1. Eight case reports included in this study met the low risk of bias category. The other three case reports met the moderate risk of bias category. Therefore, the included case reports were of high quality. High-quality case reports should describe the clinical condition, medical history, treatment history, intervention given, and post-intervention condition. Any side effects after treatment should also be addressed in the case report.
3.2. Main findings
Based on our review (Table 2), data obtained from the reviewed case reports included authors, patient’s gender, age, chief complaints, precipitating factors, concomitant condition, differential diagnosis, treatments, and treatment outcomes. The total number of articles selected and reviewed were eleven case reports with fourteen adult patients diagnosed with AO, nine female patients, and five male. Patients were in the age range between 26 – 81 years, and most cases occurred in the 30 – 60 years of age.
| JBI Checklist Questions | Rodriguez-Lozano et al. [23] 2010 | Thorburn et al. [29] 2012 |
Bhatia et al. [26] 2012 |
James et al. [24] 2013 |
Mortazavi et al. [20] 2014 |
Goel et al. [30] 2015 |
Kaku et al. [25] 2016 | Takenoshita et al. [21] 2017 |
Hegarty [27] 2017 |
Moreno- Hay et al. [22] 2018 | Suga et al. [28] 2020 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Were the patient’s demographic characteristics clearly described? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | |
| Was the patient’s history clearly described and presented as a timeline? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | |
| Was the current clinical condition of the patient on presentation clearly described? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Were diagnostic tests or assessment methods and the results clearly described? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Was the intervention(s) or treatment procedure(s) clearly described? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Was the post-intervention clinical condition clearly described? | Yes | Unclear | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | |
| Were adverse events (harms) or unanticipated events identified and described? | No | No | No | No | No | No | Yes | No | Yes | Unclear | Yes | |
| Does the case report provide takeaway lessons? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Results | 88,88% | 66,66% | 88,88% | 88,88% | 88,88% | 66,66% | 100% | 62,5% | 100% | 88,88% | 100% | |
The following complaints stated by the patients included very severe tooth pain every day, continuously for hours to days [20-22]; dull, intense, throbbing, constant tooth pain [23-28]; dull pain sometimes like stabbing or burning, intermittent [29]; acute tooth pain [30]; pain such as a tingling feeling to the touch [21]. These complaints mainly occurred in the maxilla (seven cases) [20-25], the mandible in two cases [28, 29], both maxilla and mandible in three cases [21, 29, 30], and spread to the right face in two cases [26, 27]. The affected teeth included posterior teeth in seven cases [20-22, 25, 29] and anterior teeth in four cases [21, 23, 24, 28].
Precipitating factors found in diagnosed patients included unknown factors in two cases [22, 27], psychological problems in three cases [21, 26, 29], acute lateral pontine infarction in one case [30], traumatic injury in one case [24], and the rest occurred after various dental treatments, such as implant placement [23, 28], tooth extraction [20, 29], orthodontic treatment [25], root canal treatment [21], bridge, and pulpectomy [21].
The use of antidepressants in AO management was reported in eight cases [20, 21, 24-26, 28, 29], and all of them reported significant results in treating AO symptoms for one to four months. The antidepressants used included tricyclic antidepressants (TCAs), i.e., amitriptyline, in three cases where the patient became pain-free in one to four months after treatment [21, 24, 29] and imipramine in one case where the pain decreased entirely after two months without any adverse effect [25]. Selective Serotonin Reuptake Inhibitor (SSRI), i.e., fluoxetine, was used in two cases [20, 26]; in both cases, it was combined with clonazepam. Moreover, atypical antidepressants, i.e., mirtazapine, were used in two cases [21, 28]; in one case, it was used in combination with aripiprazole. The pain decreased entirely after one to two weeks later in both cases.
| Author | Sex, Age | Chief Complaints | Precipitating Factor | Concomitant Conditions | Differential Diagnosis | Treatments | Outcome |
|---|---|---|---|---|---|---|---|
| Rodriguez – Lozano et al. [23] 2010 |
F, 62 | Continuous pain in moderate-intensity with sporadic episodes of intense dull, throbbing pain in the left maxillary canine and radiating the same head side. Intense pain lasted for several hours and occurred once a week. | Placement of dental implants | Migraines, receiving treatment for hypercholesterolemia | Postherpetic neuralgia, deafferentation pain, pain maintained sympathetically | Lyrica (pregabalin) 125 mg per day | Pain decreased two months after treatment |
| Thorburn et al. [29] 2012 |
M, 49 | Toothache in the right mandible region. The pain was sudden onset, intermittent, and increased in duration as the day progressed. | Tooth extraction (horizontally-impacted 48) | N/A | N/A | Eutectic mix of lidocaine and prilocaine (EMLA) cream | Pain decreased over the ensuing months and was completely pain-free. |
| F, 48 | Pain in the left maxillary region, dull with episodes of sharp or hot sensation, and presents most of the day but not interfering with sleep. | Psychological problem (Stress) | N/A | N/A | EMLA cream and topical capsaicin 0.025%. Amitriptyline (TCA) 75 mg each day, gradually decreased after two months |
Inadequate pain control. Pain-free after four months without any medication. |
|
| Bhatia et al. [26] 2012 |
F, 30 | Continuous dull aching pain with occasional exacerbation at the right side of the face lasted for three months. No triggering points and used to subside during sleep. Started three months ago after some family problem. |
Psychological problem | N/A | N/A | Fluoxetine 20 mg daily with clonazepam 0.25 mg and diclofenac potassium 50 mg whenever required. | The pain gradually decreased, and the patient became entirely pain-free four weeks after treatment |
| James et al. [24] 2013 |
M, 26 | Diffuse pain in the anterior maxillary region (then localised on 21 and 22 edentulous areas) | Traumatic injury | Irreversible pulpal inflammation of teeth 12 and 23 and over obturation on 11 with persistent apical periodontitis. | N/A | Amitriptyline 20 mg per day as a single dose | Pain decreased after two days and significantly diminished by the fifth day. Pain-free condition achieved after one month. |
| Mortazavi et al. [20] 2014 |
F, 30 | Severe sharp pain, continuing all day long, in the site of an extracted tooth (maxillary molar) and radiates to the surrounding area | Tooth extraction | N/A | N/A | Fluoxetine 10 mg twice a day and clonazepam 0.5 mg half an hour before sleep. The dosage decreased to 10 mg of fluoxetine, and clonazepam was continued until two weeks and decreased to 0.25 mg. |
Pain intensity decreased by 50% after two weeks, and pain-free achieved after one month. Two months and eight months follow up; the patient reported no sign of pain and discomfort. |
| Goel et al. [30] 2015 |
M, 40 | Acute onset of diffuse dental pain for the last 6 hours, restricted to right maxillary and mandible region. | Acute infarct in right lateral pons close to root entry zone (REZ) of the trigeminal nerve | Hypertensive for the past 4 years with irregular medications. | N/A | Aspirin 150 mg and atorvastatin 20 mg once a day. Combination of perindopril and indapamide (hypertension control) Carbamazepine 200 mg twice a day (for the pain) |
Pain decreased significantly after two weeks. |
| Kaku et al. [25] 2016 |
F, 33 | Impaired molar masticatory function and toothache in maxillary molars from halfway of the first orthodontic treatment | Orthodontic treatment with first premolar extraction | N/A | N/A | Imipramine hydrochloride 10 mg twice a day. One month later, the dose increased 10 mg thrice a day. The dose decreased to a daily dose of 10 mg until 1 year. |
Pain-free two months after treatment (no adverse side effects) |
| Takenoshita et al. [21] 2017 |
F, 58 | Heavy, splitting pain in the four maxillary front post-crown teeth (pressure-like pain) | Root canal treatment | Hypertension and hyperlipidaemia. | N/A | Amitriptyline 10 mg and increased the dose to 20 mg one week later. The 20 mg dose continued for four months, decreased gradually, and finally ceased after eight months. |
Pain-free after one and a half months after the first visit. |
| F, 39 | Heaviness on the right maxillary and mandible molars, face, and whole palate and throat. | Psychological problem (Panic Disorder) | Congenital deafness and irritable bowel syndrome. | N/A | Carbamazepine, gabapentin, and pregabalin Aripiprazole liquid 3 mg, reduced to 1.5 mg, gradually decreased to 0.5 mg eight months after the first visit |
Not effective Pain decreased after three weeks and improved significantly after two months. Pain-free after 13 months, and the patient stopped taking any medication. |
|
| F, 54 | Tingling sensation on the left mandible premolar and molar upon touch. The teeth have been treated, and an uncomfortable feeling on a provisional prosthesis. | Bridge placement and pulpectomy on left mandible first molar | Dysautonomia, gastritis, pyloric ulcer, and stomach polyps. | N/A | Mirtazapine 7.5 mg Aripiprazole 1 mg added. The dosage gradually increased to 30 mg mirtazapine and 2 mg aripiprazole. |
Pain decreased after two weeks, but the patient still had an uncomfortable feeling. The uncomfortable feeling decreased after twenty days. The pain and uncomfortable feeling decreased significantly. |
|
| Hegarty [27]. 2017 |
M, 64 | Persistent atypical oral and facial pain on the right side presented as a toothache that radiated from the right maxillary gingiva to the medial aspect of the nose. | Unknown | N/A | N/A | Tapentadol slow-release (SR) 150 mg twice a day and tapentadol rapid-acting film-coated tablet 50 mg orally every eight hours (if required) | The pain gradually decreased eight days to twelve months after treatment. There was an 80% improvement weekly. |
| Moreno-Hay et al. [22] 2018 |
F, 50 | Constant sharp pain in the left maxillary second premolar and second molar gingiva lasted one year, aggravated by talking and eating. | Unknown | N/A | N/A | Intravenous ketamine infusion combined with fentanyl and midazolam/diazepam (five times in seven years) with a single dose of methadone daily. Tramadol 50 mg twice a day |
Pain decreased entirely, and methadone was discontinued after ten years. |
| Suga et al. [28] 2020 |
M, 81 | Severe chronic pain on the surrounding gingiva of anterior mandible implant, symptoms occurred two to three times a month and gradually became more frequent. | Implant placement | Angina pectoris, hypertension, hyperlipidaemia, gastroesophageal reflux disease, chronic gastritis, insomnia, lumbar spinal stenosis, and prostatomegaly. | N/A | Mirtazapine 7.5 mg per day. | Improvement of sleep quality after one day, less anxious three days later. Pain decreased significantly and became pain-free one week after treatment; no recurrence after one year. There was no severe side effect, but the patient experienced dizziness, drowsiness and gained 2 kgs in the first week. |
Anticonvulsants, such as pregabalin, clonazepam, carbamazepine, and midazolam, were reported in five cases [20, 22, 23, 30]. The use of clonazepam combined with fluoxetine was reported in two cases where the pain decreased entirely after one month in both cases [20, 26]. Midazolam was combined with ketamine and fentanyl as an intravenous infusion, and the pain ceased completely. The use of anticonvulsants for managing AO symptoms has a significant impact. The pain in all cases disappeared entirely around two weeks to six months after treatment, where the pregabalin decreased the pain after two months and carbamazepine in two weeks [20, 22, 23, 30]. However, one case reported that carbamazepine, pregabalin, and gabapentin are ineffective in managing AO symptoms [21].
The use of EMLA cream was effective in one case where the patient became pain-free after a couple of months. In other cases, EMLA cream used in combination with topical capsaicin was reported to have inadequate pain control. Therefore, the treatment was replaced with an antidepressant (amitriptyline), and four months later patient felt no pain without any medication [29]. Two cases reported the use of analgesic opioids with tapentadol [27], methadone, and tramadol [22]. Methadone and tramadol were given in single doses and combined with intravenous ketamine infusion, where the patient reported complete elimination of the pain a few months after treatment [22].
Aripiprazole, an antipsychotic drug, was used to treat AO in two cases [21]. One of them was combined with mirtazapine. The use of aripiprazole was highly significant in reducing AO symptoms in about one to five months after the initial visit.
4. DISCUSSION
This literature review found that AO has various precipitating factors. AO can occur after trauma in teeth due to traumatic injury and dental treatment, such as tooth extraction, implant placement, bridge placement, root canal treatment, and orthodontic treatment. The widely accepted theory of the AO etiology is neuropathic disorder. According to the International Association for the Study of Pain (IASP), neuropathic pain is initiated or caused by the presence of primary lesions or dysfunction in the somatosensory nervous system [31-33].
Neuropathic pain indicates structural or functional abnormalities in the peripheral nervous system or central nervous system [34]. Dental treatment procedures, such as periodontal surgery, endodontic therapy, tooth extraction, implant placement, or preparation of the crown, have the potential to cause physical, chemical, and thermal trauma that can cause damage to nerve fibers [35]. The damage is likely to change nerve continuity on a tissue, resulting in the deafferentation of trigeminal sensory nerve fibers [3, 5, 31, 36]. Deafferentation of nerve fibers initiates persistent pain, paresthesia, and dysesthesia even after the wound has recovered completely [5, 36]. This explains patients' complaints of severe dental pain for hours to days with the characteristics of sharp, intense, and constant aching pain. In addition, the theory of idiopathic mechanisms can also be involved [3, 36]. Psychological problems, such as depression and anxiety, can also be predisposing factors or secondary factors of AO occurrence [5, 14, 36].
The results showed that the drugs used in AO management consist of antidepressants, anticonvulsants, analgesic opioids, antipsychotics, and topical analgesics [5, 37]. Drugs for neuropathic pain recommended by the IASP include TCA, gabapentin, pregabalin, Selective Serotonin Norepinephrine reuptake inhibitors (SNRIs) as the first line, while patch lidocaine, patch capsaicin, and tramadol as the second line [15]. In comparison, the Canadian Pain Society (CPS) recommends TCA and anticonvulsants as the first line and tramadol and analgesic opioids as the second line [17].
Antidepressants, TCAs, such as amitriptyline and imipramine, are the most frequently used drugs with amitriptyline as the mainline agent if side effects or contraindications do not cause restrictions in their use [2, 3, 5, 37, 38]. The analgesic effect of TCA works with its primary mechanism of inhibiting the reuptake of serotonin and norepinephrine neurotransmitters. In addition, TCA has also been proven to have the nature of local anesthesia effect by blocking the sodium and calcium channel and also blocking the alpha-adrenergic, H1-histaminergic, muscarinic acetylcholine, and NMDA receptors [3, 5, 14, 38]. This mechanism explains its antinociceptive effects that work quickly. Amitriptyline is prescribed in the initial dose of 10-25 mg and increases gradually to approximately 100 mg [5, 14]. The use of TCAs is limited due to their side effects, such as dry mouth, constipation, sweating, dizziness, blurred vision, sleepiness, palpitations, sedation, urine retention, and sexual dysfunction [38, 39]. TCA should not be used by the patients in the healing phase of myocardial infarction, heart conduction disorders, compensation of the heart, or porphyria [38].
Antidepressants, Selective Serotonin Reuptake Inhibitors (SSRIs), fluoxetine, can be used to treat AO, both with a single dose or in combination with other drugs. SSRI 10-20 mg per day can be prescribed if the patient has contraindications or to avoid the strong side effects from amitriptyline [36, 40]. The combination of fluoxetine and clonazepam can exert the inhibition effects of gamma-aminobutyric acid (GABA), affect serotonin and adrenaline levels in synapses, as well as inhibit spinal multi-synaptic afferent pathways [20]. Mirtazapine, the Noradrenergic and Specific Serotonergic Antidepressant (NASSA), can also be used to treat AO, especially in high anxiety and emotional patients, at a dose of 7.5 mg a day [21, 28]. Mirtazapine works by antagonising the alpha-2-adrenergic central presynaptic receptor, increasing serotonin and norepinephrine release [41]. Norepinephrine acts as a potent H1 receptor antagonist that produces sedation and soothing effects. This drug produces slightly lighter anticholinergic and cardiovascular side effects than amitriptyline with equal effectiveness [42].
Case reports reviewed in this study showed the effectiveness of anticonvulsants as membrane stabilizers in treating AO symptoms. Anticonvulsant treatment is widely recommended and has proven to successfully overcome AO complaints in several studies [2, 9, 33]. Gabapentin and pregabalin bind to alpha-2-delta subunits associated with voltage-gated channel calcium on dorsal horns, reducing calcium entry into exciting neurons. This action reduces the release of neurotransmitters, such as glutamate, calcitonin gene-related peptide (CGRP), and substance P [17, 33]. In adult patients, pregabalin (brand Lyrica) can be given with an initial dose of 150 mg divided into 2-3 doses per day with a maximum dose of 300 mg, carbamazepine 300 mg divided into 2-3 doses per day with a maximum dose of 800 mg, and clonazepam 0.25 - 1.5 mg per day with a maximum dose of 4 mg.
This study also found the use of dopamine partial agonists (DPA), such as aripiprazole, effective for AO treatment. These findings are in accordance with the results of the research conducted by Tu et al., in which aripiprazole was used, both as a single dose or combined with TCA [7]. High dopamine tonic activities can increase pain due to the reduced release of μ-opioid [43]. Therefore, aripiprazole, as a dopamine stabilization agent, can reduce pain intensity in patients diagnosed with AO [7].
Antidepressants are recommended as first-line agents and have been proven to be effective for treating various types of neuropathic pain, including pain symptoms in AO. Antidepressants have a very broad mechanism of action. Nevertheless, for the same reason, this medication has a higher incidence of adverse effects than other medications that have been studied [44]. Therefore, anticonvulsants have been suggested as an alternative for treating AO, as they have shown fewer side effects [36]. One study stated that in peripheral neuropathic pain, the efficacy of TCA is similar, if not better, compared to gabapentin and opioids, such as oxycodone or tramadol [45]. A study conducted by Tu et al. (2019) revealed the effectiveness of antidepressants and antipsychotics (DPA) combination in the treatment of AO. The results of this study also proved that the combination of TCA and DPA is efficient and produces fewer side effects; that is why it is not advisable to give TCA as a single treatment [7].
Two AO cases reported the effectiveness of analgesic opioids, such as tramadol, tapentadol, and methadone, for treating patients with neuropathic pain, which is consistent with the results of several RCTs [15, 39, 46]. However, this group of drugs is more recommended as a second or third-line of treatment because of their side effects, such as addiction, death, overdose and risk of abuse, also the complexity of monitoring and follow-up [32, 39]. Tramadol and tapentadol are both opioid agonists, but tapentadol is more potent than tramadol. An opioid is an analgesic that works centrally with two mechanisms, inhibiting nociceptive transmission in presynaptic and postsynaptic μ-opioid receptors and inhibiting the reuptake of serotonin and norepinephrine [27, 32, 33]. The use of topical drugs, such as a eutectic mixture of lidocaine and prilocaine (EMLA cream) 5% and a capsaicin 0.025%, for neuropathic pain treatment showed excellent results in some cases [3, 5]. Topical drugs, such as lidocaine and capsaicin, are recommended as second-line agents in patients with peripheral neuropathic pain [32], which might be due to their mechanism of action. Lidocaine patches or gel, for example, act locally by blocking voltage-gated sodium channels and reducing the conduction of spontaneous ectopic nerve impulses [32, 33]. However, the effectiveness of the use of tropical drugs is still not widely proven, and effective results in pain treatment are still few in numbers.
Opioids have been widely used and have been reported to have mild effects in pain management, including inhibiting nociceptive transmission. Moreover, they are quite effective compared to other drugs used for neuropathic pain and have been reported to have similar effects to antidepressants [32, 33, 39]. EMLA cream was reported to be 60% effective in reducing pain in 38 patients, whereas 0.025% capsaicin was reported to be 50% effective in reducing pain approximately three months after treatment [9]. Yet, considering that topical lidocaine cannot penetrate deeper than 8-10 mm, it is only indicated for localized neuropathic pain. Reported side effects of its use include mild local reactions, such as rash and itching of the skin. Additionally, the lack of systemic absorption of this drug may benefit elderly patients [39]. Although the pain relief effects of topical drugs are not widely proven, the minimal side effects showed the need for further research to be carried out to show the effectiveness of topical medications [9].
Limitations in this systematic literature review include that AO has various terminology. Hence the search results are less focused. In addition, the recent case reports regarding AO are limited because it is still poorly understood, and some of the existing case reports do not contain adequate information. Another essential note in this systematic literature review is that this review is conducted based on case reports showing individual treatment outcomes. Also, not all case reports describe treatment outcomes precisely in the form of a timeline. Lastly, some of the research limitations indicate the need for clinical research on the effectiveness of the drugs used as AO management through randomized controlled trials (RCT) or open-label studies.
CONCLUSION
Based on the results, pharmacological agents, such as antidepressants, anticonvulsants, antipsychotics, or analgesic opioids, are commonly used in AO management, where antidepressants seem to be the most effective ones. All case reports reviewed reported that the patient was free from pain complaints a few weeks to several months after treatment.
REGISTRATION OF SYSTEMATIC REVIEW
This systematic review has been registered on PROSPERO with register ID CRD42021245918.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the current study are available from the corresponding author [T.M], on reasonable request.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.
SUPPLEMENTARY MATERIAL
PRISMA checklist is available as supplementary material on the publisher’s website along with the published article.


