Cytotoxicity of 3D Printed Materials for Potential Dental Applications: An In Vitro Study
Abstract
Aim of the Study:
This study aims to evaluate the cytotoxicity of different stereolithographic 3D printed materials and the polyetheretherketone for varying time intervals using MTT assay and by application of these materials for intra-oral usage.
Materials and Methods:
Three groups of disc specimens (5 mm diameter, 2 mm thick) were manufactured by either selective laser melting (PA6 nylon, fiber reinforced PA6 nylon) or milling (PEEK). The cytotoxicity of these materials was tested by culturing the samples on human fibroblast cell lines prior to MTT assays (3- (4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, a yellow tetrazole). MTT assays were completed at 3, 6, and 10 days. Cell grown in normal medium without experimental material served as control. The total cell number and viability of cells pre-incubated with PA6 nylon, fiber-reinforced PA6 nylon, and PEEK were comparable to the control samples. Differences between the growth inhibitory effects of the samples in the MTT assay were below 0.05%.
Results:
Both nylon and fiber-reinforced nylon reduced the proliferation of normal human fibroblasts up to 6 days of treatment. PEEK had better biocompatibility than PA6 nylon and fiber-reinforced nylon. Both PA6 nylon and fiber-reinforced are 3DPrinted materials that showed cytotoxicity at 10 days. However, soaking the nylon materials for 6 days made them safe on normal human cells.
Conclusion:
PEEK material can be considered for intraoral usage as the material is biocompatible. Both PA6 nylon and fiber-reinforced PA6 nylon materials showed increased in-vitro cell death at 10 days, suggesting that they are non-biocompatible for intraoral usage beyond 6 days.
1. INTRODUCTION
There has been a rapid increase in the use of Computer-Aided Design (CAD)/ Computer-Aided Manufacture (CAM) and Additive Manufacture (AM) in dental applications over the past decade. Polyether Ether Ketone (PEEK) has become a common material for dental appliances produced using CAD/CAM. PEEK is a white, radiolucent, rigid material [1]. It is nonallergic [2, 3], resists hydrolysis, non-toxic and is highly biocompatible [4, 5]. A study conducted in 2015 showed that PEEK has many advantageous properties that make it suitable for use as an esthetic metal-free orthodontic wire [6] and space maintainers such as lingual arch, a band with loop, and removable plate [7]. Maekawa et al. (2015) have shown that PEEK has many valuable properties useful for esthetic metal-free orthodontic wire despite lower esthetic properties [6].
Nylon is one of the most important industrial polymers [8]. Nylon PA6 can be produced using various AM processes that offer increased design freedom over CAM. The numerical nomenclature for nylon is derived from the number of carbon atoms in the diamine and dibasic acid monomers used to manufacture it. Nylon 6 is used in a wide range of industries and products, including toothbrushes and engineering plastics [9]. Nylon 6 can be extruded (melted and forced through a nozzle) and is, therefore, a good plastic for both injection molding and 3D printing. In addition, Nylon is considered a metal replacement because it can create parts that are durable, strong, and lightweight. Nylon 6 is most commonly available in black, white, and its natural color. Its good biocompatibility has further led to its implementation in medical applications, such as providing a scaffold for tissue cultures [10], foil for orbital implants, bone support in arthroplasty [11] and cellular control devices in molecular medicine [12]. However, their use for intraoral applications is under investigation.
Biocompatibility and mechanical properties are the most important considerations for orthodontic clinical use in the 3DPrinting materials. Pandey et al. (2016) stated that biomaterials must be proven safe for long-term contact with human tissues without causing rejection reactions. For a material to be biocompatible, it must be non-toxic, non-immunogenic, non-mutagenic and non-carcinogenic [13]. PEEK has been proven safe for the production of both intra-oral and implantable devices. The biocompatibility and safety of nylon for intra-oral use have been less widely reported.
The aim of this present study is to evaluate the cytotoxicity of stereolithographic
3D printing materials for varying time intervals using MTT assay (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, a yellow tetrazole) and application of these materials for intra-oral usage.
2. METHODS
This study was a comparative study on biocompatibility between PEEK produced using CAM and PA6 nylon materials produced using Additive Manufacturing (AM). Cell culture studies are usually the primary point when evaluating biocompatibility, providing an examination of toxicity in a simplified system that minimizes the effect of confounding variables [14]. By using cells from murine or human fibroblast cell lines, the cytotoxicity of various materials can be determined [15]. This study used human fibroblasts, breast origin (primary fibroblast cells ATCC PSC 201-202).
2.1. Sample Preparation
2.1.1. PA6 Nylon
Five discs of nylons (5 mm radius and 2 mm thick for fiber reinforce PA6 Nylon, and 2.5 mm radius and 2mm thick for PA6 Nylon) were designed using Magics software (version 20, Materialise, B). The specimens were fabricated directly onto the build plate in 0.125mm thick layers using a Mark Two machine (MarkForged, USA) in a proprietary PA6 nylon material. Elmer's Washable School Glue (Elmer's Products, Inc, USA) was used as a build plate adhesive. Following completion, the samples were removed from the build plate, rinsed on hot water, and scrubbed to remove visual traces of the adhesive, then soaked in isopropyl alcohol for 10 minutes before being air-dried. They were then heat-sealed in a polythene bag prior to being dispatched for testing.
2.1.2. PEEK
Two different dimensions of PEEK samples were prepared by CAM /milling. Five specimens (2.5 mm radius and 1 mm thick) were named small PEEK samples, and five samples (5mm diameter and 2 mm thick) were named large PEEK samples that were fabricated. After fabrication, the disc samples were treated in the same way as the PA6 Nylon samples.
2.1.3. MTT Assay
The MTT method focuses mainly on the mitochondrial function (dehydrogenase activity). Materials (Table 1) were placed in a 12-well plate and soaked in 70% ethanol that was left to evaporate overnight in a biological safety cabinet. The next day, a total number of 2.5×105 cells were seeded on top of the materials and incubated at 37°C and 5% CO2. Three plates to be read within 3, 6 and 10 days were prepared. At a designated period, media was withdrawn, and a total concentration of 0.5 mg/ml of MTT dissolved in the media was added to cells. MTT was kept for 4 hours, and then media was removed, and isopropyl alcohol was added to dissolve formazan that formed from MTT utilization by cells. The materials were then removed from plates and the plates were read using a plate reader (Biotek, ELISA, USA) at a wavelength of 490 nm to measure absorbance of formazan dissolved in isopropyl alcohol.
| Material type | Color |
|---|---|
| No testing material | Control |
| PEEK | Gray small |
| PEEK | Gray Large |
| PA6 Nylon | White |
| Fiber reinforced PA6 Nylon | Black |
3. RESULTS
The results of the MTT assay at 3, 6, and 10 days are shown in Figs. (1-3), respectively. In the MTT assay, the presence of the inhibition zones marked by the loss of the red dye indicates cell death. In addition, losing red dye by a given cell indicates cell death. Inhibition zones were absent in the control group and experimental groups at all examined time points (P-values > 0.05). Furthermore, 100% of the cells retained the red color dye. Thus, both PA6 Nylon and PA6 Nylon seemed to be non-cytotoxic as examined using MTT assay. The results also showed that the PA6 Nylon and fiber-reinforced PA6 Nylon reduced the proliferation of normal human fibroblasts at 6 days of treatment, but this effect was not observed at 10 days.
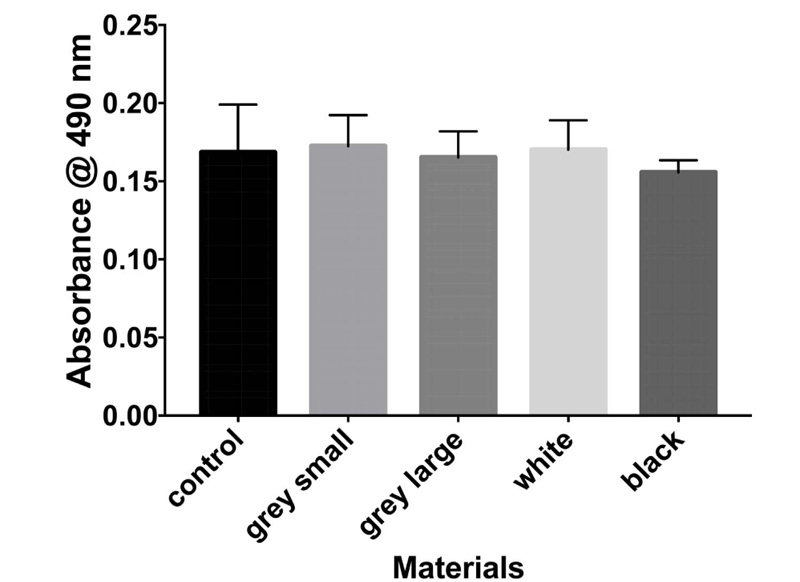
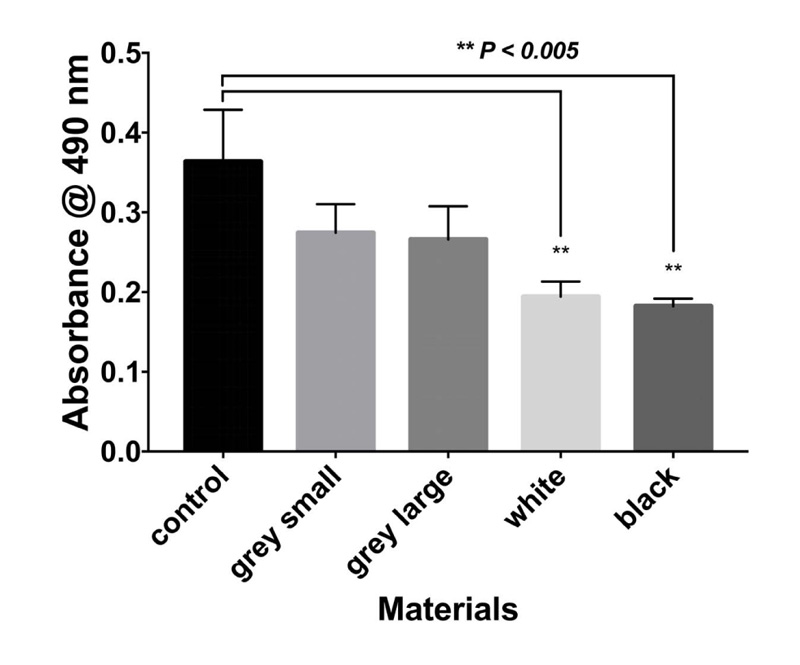
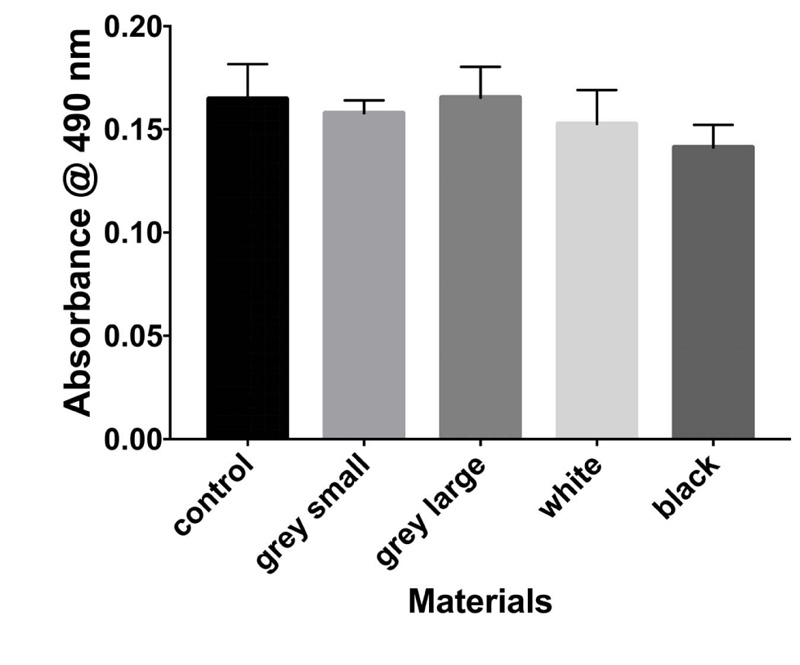
The result of biocompatibility showed that all materials tested fulfilled the acceptance criteria for some parts of ISO 10993. Cytotoxicity testing showed that the two PA6 Nylons (used in the MarkForged, Mark two production process) are nontoxic.
The effects of PA6 Nylons and fiber-reinforced PA6 Nylon on human fibroblasts were similar. The latter showed that the leachable substances are in compliance with allowable limits for nonvolatile residue, residue on ignition, heavy metals, and buffering capacity. PEEK has been reported as biocompatible material. Soaking the materials in water at 6 days enabled the PA6 nylon to be a safe material for use.
4. DISCUSSION
In the current study, cytotoxicity of PA6 nylon and fiber-reinforced PA6 Nylon was examined using MTT assay. The results showed that both PA6 Nylon and PA6 Nylon are non-cytotoxic. Nylon 6 is a synthetic thermoplastic linear polyamide that was first produced in 1935 by American chemist Wallace Carothers who then worked at the DuPont research facility in Delaware. Nylons tend to absorb moisture due to the amide chemical group and this is considered a disadvantage. Moisture uptake also has a huge influence on dimensional variations; this must be considered when designing parts.
The results showed that both types of nylons reduced the proliferation of normal human fibroblasts (of breast origin) at 6 days of treatment, but this effect was not observed at 10 days. Thus, soaking the two nylons in water for 6 days might make them more biocompatible. Further material testing would be required to determine whether the nylon would be safe for long-term use in the oral cavity. Nylons have high tensile strength and elasticity and are very resistant to abrasion and chemicals [16]. Due to its design flexibility and lightweight, this could make this material extremely useful in a broad range of dental applications, including space maintainers, occlusal appliances, orthodontic, and sleep apnea devices [16].
Studies that examined the cytotoxicity of nylons PA6 are limited. Using MTT assay and RT-PCR for tumor necrosis factor-alpha (TNFα) and interleukin 6 (IL-6) osteolysis markers, Firouzi et al., found high cell viability of nylon 6 coated ultra-high molecular weight polyethylene [17]. The study concluded that nylon could be used as a novel material in clinical applications with lower cytotoxicity, and less wear debris-induced osteolysis [18]. Similarly, coating dimethoxybenzidine (DMOB) with nylon 6 significantly reduced the cytotoxicity of DMOB to mouse cultured fibroblast cells by about 78% [12, 19]. The result of this study confirms these findings and can be considered in the context of a limited number of the materials used in the research.
One limitation of the current study is the use of a single assay – the MTT assay- to assess the safety of the material used. It is to be noted that the current study is considered a proof of concept where more comprehensive safety studies are warranted.
CONCLUSION
Based on the results of the MTT tests employed in this study, it can be concluded that the nylons routinely in use in AM technologies such as SLM do not exhibit cytotoxic potential at 3 and 10 days, and for this, soaking is required for 6 days. Further clinical trials should be performed to show the in vivo behavior of this nylon under oral environmental conditions.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data supporting findings of this study is available from corresponding author [N.A.A-.M], upon reasonable request.
FUNDING
This research was funded by the Deanship of Research at Jordan University of Science and Technology, Irbid, Jordan.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


