Is the Mandibular Condyle Involved in Medication-Related Osteonecrosis of the Jaw? Audit of a Single Tertiary Referral Center and Literature Review
Abstract
Background:
Medication-related osteonecrosis of the jaw (MRONJ) may manifest as exposed mandible bone. Recent reviews of the incidence of MRONJ report primarily as exposed cortical bone of the mandibular body, ramus, and symphysis with no reports of condylar involvement.
Objective:
The aim of this study is to analyze the topographical incidence of MRONJ, comorbidities, demographics data, and clinical characteristics of patients diagnosed with MRONJ between 2014 and 2019 in the Maxillo-Facial Surgery Department University of Campania “Luigi Vanvitelli”, and compare these results with published reports.
Methods:
Data on 179 patients were collected for the study, including gender, age, underlying malignancy, medical history, and specific lesion location-identifying premaxilla and posterior sectors area involvement for the maxilla and symphysis, body, ramus, and condyle area for the mandible. A literature review was performed in order to compare our results with similar or higher sample sizes and find if any condylar involvement was ever reported. The research was carried out on PubMed database identifying articles from January 2003 to November 2020, where MRONJ site distribution was discussed, and data were examined to scan for condylar localization reports.
Results:
30 patients had maxillary MRONJ, 136 patients had mandibular MRONJ, and 13 patients had lesions located in both maxilla and mandible. None of the patients reported condylar involvement, neither as a single site nor as an additional localization. Literature review results were coherent to our findings showing no mention of condylar MRONJ.
Conclusion:
Results do not show reports of condylar involvement in MRONJ. Although the pathophysiology of the disease has not been fully elucidated, two possible explanations were developed: the first one based on the condyle embryogenetic origin; the second one based on the bisphosphonate and anti-resorptive medications effects on the different vascular patterns of the mandible areas.
1. INTRODUCTION
Medication-Related Osteonecrosis of the Jaw (MRONJ) is a rare but severe condition characterized by non-healing exposed bone in patients that were or are currently using an antiresorptive or antiangiogenic drug and have no history of the previous radiotherapy to the head and neck area. The diagnostic criteria for MRONJ developed in 2006 by Ruggiero et al., then adopted by the American Association of Oral and Maxillofacial Surgeons (AAOMS) and further updated in 2014, are based on pharmacological history as well as clinical features [1-3]. MRONJ surgical management should be performed through digital planning and using less invasive instruments, such as piezoelectric surgery and low-level laser therapy, which are described to minimize structural and vascular damage to the bone and promote a faster recovery of both soft and hard tissues [4-7]. These guidelines focus on the clinical and pathological features of MRONJ and represent the current standard staging and treatment.
During our practice, our team treated a Stage III MRONJ of the right mandible, where the extensive disease led to a right Hemi-mandibulectomy. Although a sub-condylar fracture was observed, the condyle histological analysis resulted in being free of disease. This outcome was the primum movens of our study, causing us to question if the condylar area was ever involved in MRONJ.
According to recent reviews, 64-70.6% of MRONJ manifest as exposed mandibular bone, the maxilla is involved in 18.3-27% of cases, and osteonecrosis is present in both jaws in 9-11.1% of cases [8, 9]. None of these studies reported condylar involvement, neither as a single site nor as an additional location.
Although the first MRONJ case was reported in 2003, the mechanisms underlying MRONJ pathogenesis have been proven difficult to unveil and represent a challenge to the specialists involved [10]. The main pathogenic hypotheses involve suppressed or deleted bone turnover, cellular toxicity, infection, and the inhibition of angiogenesis [11, 12]. Among these theories, the suppressed bone turnover is a widely accepted pathophysiological explanation [13]. BP and other antiresorptive drugs like denosumab inhibit osteoclast differentiation and function, thus increasing apoptosis, decreasing bone resorption, and remodeling capabilities. Authors have described how reduced bone resorption followed by a reduction in bone formation can lead to tissue micro-damage and necrosis [14]. Among risk factors, the preexistence of dental or periodontal infection in patients under BP or antiresorptive medications represents a well-known precipitating event to MRONJ development [11]. Dentoalveolar surgery was identified as an important MRONJ risk factor, with 52-61% of patients reporting teeth extractions as a trigger event. Nevertheless, studies showed that it is not a prerequisite for the pathology onset [11, 15]. None of these mechanisms would seem to explain the absence of condylar involvement.
The aim of this study is to determine if the condyle is ever involved in MRONJ retrospectively investigating causes, demographics, and characteristics of patients diagnosed with MRONJ between 2014 and 2019 in the Maxillo-Facial Surgery Department of the University of Campania “Luigi Vanvitelli”, confronting our results with literature reports.
2. MATERIALS AND METHODS
An audit of patients diagnosed with MRONJ who attended the Maxillo-Facial Surgery Department University of Campania “Luigi Vanvitelli” from January 2014 to March 2019 was conducted. This study was conducted in accordance with the ethical principles of the Declaration of Helsinki (2002) and approved by the local ethical committee (prot. N°319, 23/10/2020).
The patients’ data were anonymized when extracted from the clinical database. The inclusion criteria were: oncologic patients, use of antiresorptive drugs, and surgical management. The exclusion criteria were a history of head and neck radiation, a metastatic bone disease of the maxillofacial region. Data including gender, age, underlying malignancy, medical history, and lesion location were collected. Specific sub-locations areas were identified in the maxilla (as premaxilla and posterior sectors) and the mandible (as symphysis, body, ramus, and condyle).
The radiological analysis was performed through orthopantomography (OPT), and Cone Beam Computed Tomography (CBCT) scans. MRONJ was staged according to AOOMS 2014 classification. After surgery, all harvested specimens were sent to pathological anatomy. All collected bone specimens were fixed in formalin (10% neutral buffered) and subjected to decalcifying treatment with Electrolytic Decalcifying Solution (based on hydrochloric and formic acid). Subsequently were paraffin-embedded, sectioned (5μm slides), and stained in Hematoxylin and Eosin. An experienced Pathologist analyzed all the slides.
A literature review was performed in order to compare our results with similar or larger sample sizes and find if any condylar involvement was ever reported. The research was carried out on the PubMed database, identifying articles eligible for review from January 2003 to November 2020. The search was conducted up to November 2020. Articles language was limited to English using database supplied filters. Study designs included retrospective studies, case series, and reviews. The keywords were used and combined with Boolean operators as follows: BRONJ OR osteonecrosis, BRONJ OR osteonecrosis AND jaw, BRONJ AND localization, osteonecrosis AND localization, bisphosphonates and medication-related osteonecrosis of the jaw AND localization, (MRONJ OR BRONJ) AND condyle. Articles were considered eligible if MRONJ site distribution was described in the text, tables, or graphs, and data were examined to scan for condylar localization reports.
3. RESULTS
179 patients met the criteria of this retrospective study. This included 138 women and 41 men. The age of the patients ranged from 56 to 78 years (mean 66.25 ± 9.58 years). Breast carcinoma patients were mostly represented (52.4%), followed by prostate carcinoma (21.2%), multiple myeloma (20.5%), lung carcinoma (4.3%), and kidney carcinoma (1.6%). No patient was diagnosed as Stage 0, 66 patients as Stage I, 108 patients as Stage II, 5 patients as Stage III. Most patients, 107 (59.8%), were treated with nitrogen-containing BP, 55 (30.7%) had a history of denosumab intake, and 17 (9.5%) patients reported a subsequent or alternating intake of BP and denosumab (Table 1).
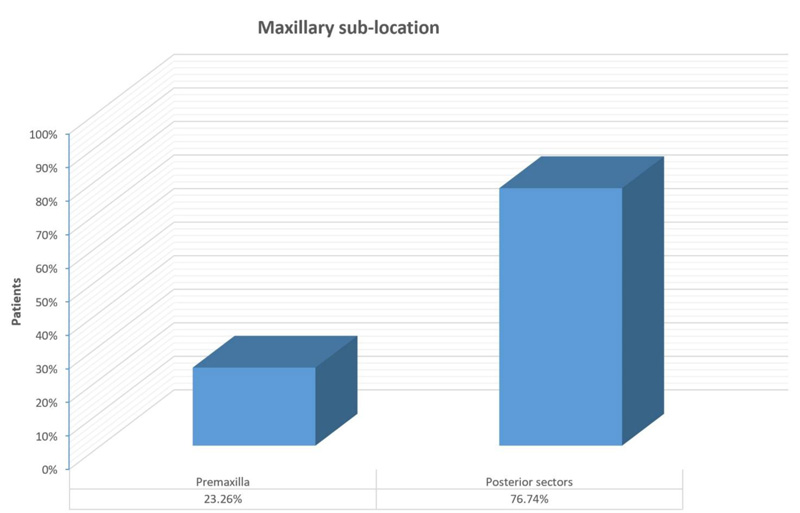
Concerning site distribution, 136 patients (75.9%) had mandibular involvement, 30 patients (16.9%) had maxillary involvement, and 13 patients (7.2%) had lesions located in both the maxilla and mandible bones. As regards maxillary bone, 10 cases (23.26%) were localized in the premaxilla, 33 (76.74%) in the posterior sectors (Fig. 1).
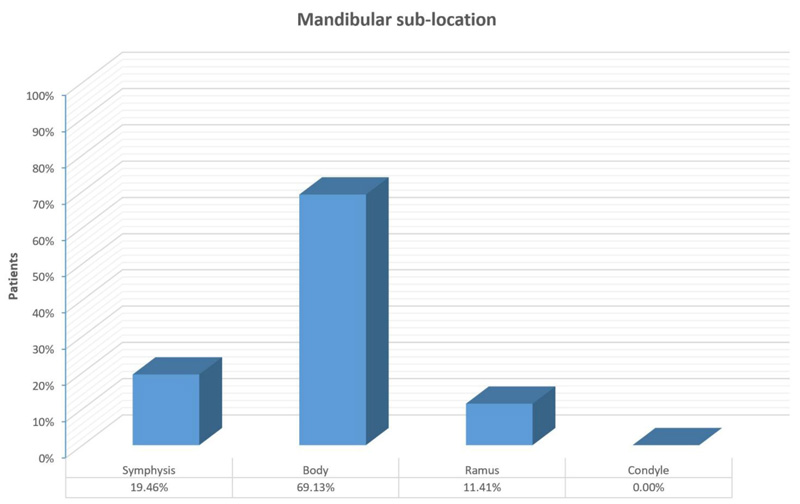
As regards the mandible, symphysis was involved in 29 cases (19.46%), body in 103 (69.13%), ramus in 17 (11.41%). No report of condylar involvement was shown, neither as a single site nor as an additional location (Fig. 2).
| PATIENTS | ||
|---|---|---|
| Sex (female, %) | 138 (77.1%) | |
| Sex (male, %) | 41 (22.9%) | |
| Age (years, mean ± SD) | 66.25 ± 9.58 | |
| Location | ||
| Mandible, n (%) | 136 (75.9%) | |
| Maxilla, n (%) | 30 (16.9%) | |
| Mandible & Maxilla, n (%) | 13 (7.2%) | |
| Mronj Stage | ||
| Stage 0, n (%) | 0 (0%) | |
| Stage I, n (%) | 66 (36.8%) | |
| Stage II, n (%) | 108 (60.4%) | |
| Stage III, n (%) | 5 (2.8%) | |
| Underlying Malignant Neoplasm | ||
| Prostate Carcinoma, n (%) | 38 (21.2%) | |
| Breast Carcinoma, n (%) | 93 (52.4%) | |
| Multiple Myeloma, n (%) | 37 (20.5%) | |
| Lung Carcinoma, n (%) | 8 (4.3%) | |
| Kidney Carcinoma, n (%) | 3 (1.6%) | |
| Antiresorptive Agents | ||
| Nitrogen-containing bisphosphonates, n (%) | Alendronic acid + cholecalciferol | 14 (7.9%) |
| Zoledronic acid (monohydrate) | 93 (51.9%) | |
| Denosumab, n (%) | 55 (30.7%) | |
| Alternating Bisphosphonates and Denosumab intake, n (%) | 17 (9.5%) | |
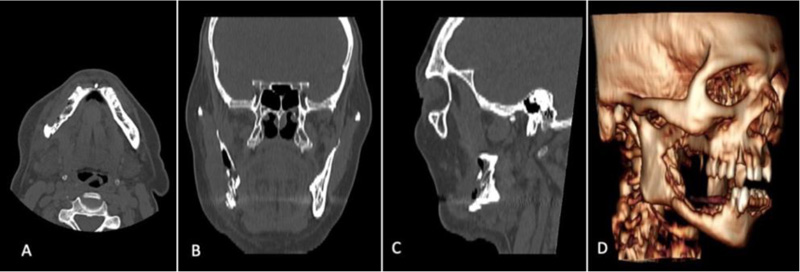
None of the condylar specimens harvested during wide mandibular resection showed any morphologic alteration consistent with necrosis. We report one emblematic case, a Stage III MRONJ of the right mandible, where we performed a right Hemi-mandibulectomy from the horizontal branch of the mandible to the right condyle due to a sub-condylar pathologic fracture (Figs. 3 and 4).
The histological sections of the mandibular condyle showed no significant morphological alterations (Fig. 5).
4. DISCUSSION
In this retrospective analysis, we investigated the MRONJ lesions of 179 patients emphasizing their occurrence and location. 136 patients with mandibular MRONJ underwent surgical treatment, but none of them included condylar involvement even in severe advanced stages.
Histologically 4 layers are present in condylar morphology: 1) connective tissue (fibrous joint layer), 2) undifferentiated mesenchymal layer (proliferative), 3) transitional layer, and 4) hypertrophic cartilage layer [16]. Osteonecrosis shows principally necrotic spots of non-mineralized tissue, areas of empty osteocytic lacunae, the presence of resorption pits with rare osteoclast-like cells, and the presence of bacteria and inflammatory infiltrate [17]. Other common features usually include bone architecture loss, the absence of a proper Haversian system, or proper marrow spaces. None of the condylar specimens analyzed showed any of these features or any other alterations related to therapy.
Therefore, a literature review was performed in order to compare our results with similar or higher sample sizes and find if any condylar involvement was ever reported. Despite the extensive literature available, most authors focused on the differences between maxillary and mandible bone, reporting mean values of 58-71% for the mandible, 18-36% for the maxilla, and 3-11% for both jawbones [8, 11, 15, 18-21]. The prevalence reported in our results is in line with the data reported in the literature, slightly higher for the mandible (75.9%) and slightly lower for the maxilla (16.9%) (Table 1).
Otto et al. in 2012 determined the localization prevalence, reporting 70.6% in the mandible, 18.3% in the upper jaw, and 11.1% in both jaws. Interestingly, they graphically expressed the distribution of these lesions, showing a predilection for the molar and premolar areas [18].


Campisi et al. in 2014 reported how the mandible is more frequently affected than the maxilla, expressing their concerns on the presurgical evaluation of the true extension of the necrotic bone due to the lack of radiographical data [11].
Khan et al., on behalf of the International Task Force on Osteonecrosis of the Jaw in 2015, reported a prevalence of 65% for the mandible, 28.4% for the maxilla, and 6.5% for both mandible and maxilla. It should be noted that 0.1% was reported for “other locations”, although these are not specified [19].
AlDhalaan et al. in 2020 reported how the mandible is more likely to develop osteonecrosis (73%) than the maxilla (22.5%) since the single blood supply of the mandible makes it more prone to necrosis and infections [21].
Literature research did not report any case of condylar MRONJ neither as a single site nor as additional localization, and additional analysis on temporomandibular disorders prevalence and epidemiology show how MRONJ was never cited as the cause of disease [22-24]. The sample case we described, where the patient’s disease extended from the right canine to the right subcondylar area, led us to investigate why the MRONJ did not advance further to the condyle and if single site involvement of the condyle ever occurred during our practice (Figs. 1-3). Therefore, we developed two hypotheses that might explain the absence of condylar involvement in MRONJ: the first one was built on the different embryogenetic origin of the condyle compared to the rest of the mandible; the second one was based on the effects of BP and anti-resorptive medications on the different vascular patterns of the mandible components [25, 26].
Our first hypothesis is supported by the embryological findings in condylar development and remodeling ability.
The development of the mandibular bones occurs in different ways along the proximal-distal axis: the distal region undergoes endochondral-like ossification to form the symphysis, the middle and largest part undergoes intramembranous ossification while the proximal region of the mandible is histologically classified as secondary cartilage and develops from endochondral ossification. According to some authors, the condylar cartilage represents a growth center and plays a part in jaw growth [25]. Obwegeser hypothesized the coexistence of two growth centers at the condylar level: one responsible for the longitudinal growth of the jaw, the other for the three-dimensional growth [27]. This peculiar morphogenesis manifests itself in the condylar influence in mandible growth, the pathogenesis of dentoskeletal deformities, and in its adaptive remodeling ability following orthognathic surgery [28, 29]. Therefore, the different ossification mechanisms, multiple growth centers, and plastic remodeling ability could make the condyle less susceptible to inflammatory triggers and capable of responding appropriately to necrotic insults, possibly explaining the absence of osteonecrosis diagnosis in this case area.
The second hypothesis is built on the vascular effects of BP and anti-resorptive medications with attention to the vascular anatomy of the mandible.
Blood perfusion to the mandible should be decreased in patients on BP therapy since bone remodeling suppression should lower the metabolic demand. The reduced blood flow would lead to vascular remodeling, making the vessels smaller and consequently less able to adjust promptly to higher skeletal perfusion demands due to inflammation or infections [30].
The anti-angiogenetic effect of BP has been studied thoroughly [31, 32]. Allegra et al. described a reduced number of circulating endothelial cells progenitors and amplified endothelial cell apoptosis in myeloma patients treated with BP, hence leading to anti-angiogenesis and avascular necrosis [31]. In-vitro and in-vivo studies have suggested that BP might inhibit IGF-1 induced activation of PI3K/Akt/mTOR pathways and pursue an anti-angiogenic action via inhibition of IGF-1-induced VEGF expression and HIF-1-alpha protein accumulation in MCF-7 cells [32]. Furthermore, Ferretti et al. analyzed how zoledronic acid could perform an anti-angiogenic effect by inducing a transitory reduction in VEGF, FGF-2, and MMP-2 [33].
Few animal studies confirmed the pharmacological effects of BP on condyle bone, influencing its structure and ossification, and therefore it would be expected to find reports of MRONJ with condylar primary localization or continuity from the mandible ramus. The explanation behind this exception may reside in the condyle vascular network [34, 35].
Two main arterial sources for the condylar process are described: the former originating from the vascular network of the temporomandibular capsule, the latter originating from the lateral pterygoid muscle arteries [26]. Toure clarified the contribution of each arterial branch to the periosteal vascularization of the condyle [36]. The author highlighted three constant sources for the condyle: the superficial temporal artery, the posterior deep temporal artery, and the arterial branches to the lateral pterygoid muscle arising from the maxillary artery. He concluded that the condyle is located in an arterial circle formed by the superficial temporal artery, the posterior deep temporal artery, the transverse facial artery, the maxillary artery, and the masseteric artery. Wysocki identified the intramedullary ascending branch from the inferior alveolar artery as the most consistent source of condyle vascularization, further stressing the joint capsule contribution and reporting an endosteal blood supply of the condylar neck vessels penetrating directly into the bone [37]. This observation was also described by Olivetto et al., providing arguments in favor of the vascular independence of this region compared to the rest of the mandible [38].
According to our observations and literature analysis, the absence of an alternative vascularization of the horizontal and angle area of the mandible could make the bone tissue more susceptible to hypoperfusion and subsequent necrosis compared to the condyle area. The overall reduced blood flow related to the medication effects would be, in fact, compensated by multiple vessels, supporting the increased metabolic demand. The blood supply protection on necrosis and infection was also hinted by AlDhalaan et al. in 2020 regarding mandibular vs. maxillary MRONJ incidence and could explain why the condyle region, with its specific, independent, and multi vascular pattern compared to the rest of the mandible, is rarely affected by MRONJ as described for post-radiotherapy and post-traumatic necrosis [21, 39].
Several cases of MRONJ are preceded by exposure of the involved bone to the oral cavity by dentoalveolar surgery or trauma, and this is unlikely to happen in the condylar area. Nevertheless, these conditions are not a prerequisite for the pathology onset, and for this reason, we cannot consider the condyle exempt from the development of MRONJ with certainty [11, 15].
In oncologic patients treated with BP, the concurrence of malignancy and osteonecrosis within the same site of the jaw should not be an unexpected event. However, both clinical and imaging data lack clear pathognomonic features for one condition or the other. Ergo, the presence of localized bone sclerosis or radiopaque/radiolucent lesions in the condylar area should lead the physician towards a diagnosis of bone metastasis [40].
CONCLUSION
Results do not show reports of condylar involvement in MRONJ. Although further studies are needed to corroborate our findings, a focus on this peculiar observation may open new inquiries on MRONJ pathophysiology.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the local ethical committee (prot. N°319, 23/10/2020).
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures were followed in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
The patients’ data were anonymized when extracted from the clinical database.
STANDARDS OF REPORTING
STROBE guidelines and methodologies were followed for this study.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article is available by the corresponding author [G.L.G], upon reasonable request.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


