All published articles of this journal are available on ScienceDirect.
Comparison of an Air-Fed Mask System with Hospital-Issued Personal Protection Equipments for Dental Aerosol Protection During the COVID-19 Pandemic
Abstract
Aim:
A pilot study was conducted with the aim of developing a system to protect the eyes, nose, and mouth from the aerosol generated from a high-speed dental handpiece during the COVID-19 pandemic.
Background:
The SARS-CoV-2 virus is known to be present in the saliva of an infected individual during the contagious viral shedding phase of the disease. The use of rotary dental instruments places oral health practitioners at risk of contracting COVID-19 from infected individuals. In particular, it is very difficult to protect the mucous membranes of the face against the extremely fine aerosol produced from a high-speed dental handpiece.
Objectives:
This study aimed to develop and test a novel PPE system for use during the COVID-19 pandemic. An air-fed spray-painting mask was used under a plastic hood to protect against the aerosol from a high-speed dental handpiece. This was found to be superior compared to hospital-issued N-95 masks and eye protection in our test model.
Methods:
Subjects donned various forms of PPE whilst using a high-speed dental handpiece in a confined cubicle. The efficacy of each form of PPE was evaluated by adding fluorescein to the water coolant supply line of a high-speed dental handpiece before checking for facial contamination with an ophthalmology slit lamp.
Results:
Under our test conditions, the N-95 mask did not prevent nasal and mouth contaminations, but the combination of an air-fed mask with a sealed hood prevented these contaminations. Although goggles worn tightly did prevent contamination, the air-fed mask system was far more comfortable and did not fog up.
Discussion:
Under the rigorous test conditions of our model, we found hospital-issued PPE ineffective. We also found the single strategy of using positive airflow into a face mask ineffective, even with extremely high levels of airflow. Complete protection was only achieved reliably by the combination of physically sealing off the face from the surrounding airspace and using the air-fed system to provide an external source of air to breathe. We effectively made the clinical equivalent of a dive bell helmet. The air-fed mask is supplied by a standard dental air compressor and is simple to install for someone familiar with the technical aspects of compressors. The compressor does not rely on a filter and proves effective with cheap and easily accessible disposable items.
Conclusion:
Under rigorous testing conditions, the developed air-fed mask system with a sealed hood on low flow performed better than hospital-issued PPE against high-speed dental aerosol protection. The developed system protects the operators from the air of the room contaminated with aerosol and brings in safe air from the outside for them to breathe.
1. INTRODUCTION
This was a pilot study to prove in principle the efficacy of an industrial aerosol protection system modified to clinical use in dentistry. It was initiated as the SARS-CoV-2 virus started to spread to our region, and our access to specialised personal protective equipment (PPE) was restricted. In order to limit the close contacts of the individuals involved in the study, the participants were selected from within the practice team and also from the close household contacts of the clinical team. Our country’s workplace regulator was informed of our activity, and informed consent was gained from the study’s participants.
As PPE became available, we developed a test to compare the efficacy of various forms of PPE. The PPE issued to us by the hospital includes protective goggles, an N-95 mask, and a disposable waterproof gown. We found both our initial air-fed system and the hospital-issue PPE afforded insufficient protection from a rigorous dental aerosol challenge. We then modified our system to achieve complete protection.
Our final development performed better than an N95 mask and goggles in our test conditions and allowed spectacles or loupes to be worn without fogging up. The protection provides a physical barrier. The operator breathes in the outside air rather than being reliant on any filter. It is comfortable to wear, and a headlight can be added. It uses products and materials that are readily accessible and does not necessarily require hospital-issued PPE. After the expense of the initial set-up, the ongoing disposable costs are very low.
We continue to use this novel system in our clinic for undertaking both dentistry and oral surgery during the COVID-19 pandemic. We have made this workable, and we feel safer and more comfortable using it than a hospital-issued PPE. This may have applications for other health workers and specialties.
2. METHODS
2.1. Study Model
The efficacy of various forms of PPE was tested in a shower cabinet sealed with plastic sheeting (Fig. 1). Participants in the study donned various forms of PPE, then a high-speed dental handpiece on a piece of wood was used with fluorescein added to the water coolant supply. The participant used the handpiece on different surfaces of the wood, moving the angle of the drill every 30 seconds. This was done to simulate a clinical situation. The participant used the handpiece for 10 minutes when most of the prepared 500mls of fluorescein-dyed water had been used. The PPE was carefully doffed to avoid removal contamination before the eyes, nose, and lips were screened with an ophthalmology slit lamp. A Wood’s lamp was also used to observe the head and neck without magnification.
2.2. Fluorescein Sodium
A 1mg/ml concentration of fluorescein sodium was placed into the reservoir bottle of a dental cart supplying an air-driven high-speed dental handpiece [1]. This was prepared by adding 5ml of 10% fluorescein sodium injection (Alcon Laboratories Australia Pty Ltd) to 495ml of distilled water.

2.3. Air-Fed Mask
The air-fed mask (Anest Iwata Corporation, Japan) has a waist belt connecting the compressor and mask (Fig. 2). The air was provided by two dental compressors connected in series (Cattani S.p.A. Italy: K300x2), each of which can generate 230l/min at 5 bar. The manufacturer recommends the air consumption of the masks to be between 140 and 300l/min at 5 bar. For these experiments, the air consumption of the mask was independently confirmed by an in-line flow meter (Certifier FA Plus, TSI Corporation USA). This set-up is effectively a variant of positive air pressure respirators (PAPR) that are currently used in clinical settings. Our air-fed system differs from other PAPR’s in that outside air is breathed, and it does not rely on a filter in the contaminated room air.
2.4. Aerosol Generation
The aerosol was generated by a dental cart (Adec Performa) and a high-speed dental handpiece running between 180,000-220,000rpm (NSK S Max 600, Nakanishi Inc, Japan) using a cylindrical diamond bur (Mani SF-41ISO 109/010). The participants were asked to hold the handpiece and a small block of wood at their natural working distance (about 300-400mm) to mimic operative dentistry. To avoid non-experimental contamination, each participant involved in each experiment had not previously been in the test room that day.
2.5. Breathable Air Generation
A breathable air quality hose was used (Esdan, Australia). The air from the compressor first passed through a three-stage breathing air filter (Festo Group, Esslingen am Neckar, Germany). The airline was then split into 3 hoses to use 3 air-fed masks. One of these masks was used in the trials. The air entering the mask was monitored for carbon monoxide (Gas Alert Micro Clip, Honeywells, USA).

2.6. Examination of Fluorescein Contamination
Fluorescein maximally absorbs light at approximately 493nm and emits a greenish light at approximately 520nm. Cobalt blue light provides a suitable means of exciting sodium fluorescein [2, 3]. Immediately following each test, the protective equipment was removed, and the participants were examined. The head and neck were examined in a dark room with a Wood’s lamp for gross contamination. Microscopic contamination was then determined under slit-lamp examination by an ophthalmologist. Slit-lamp microscopy was used with a Takagi SM-70N slit-lamp microscope. Examination of test participants was performed at 10x, 16x, 25x, and 40x magnification. The examination included identifying the presence of fluorescein droplets using plain illumination and using a cobalt blue filter. Examination of the corneas, tarsal and bulbar conjunctiva, and eyelid margins was performed. Slit-lamp examination of the anterior nares and lips was also performed. The presence of fluorescein was documented and photographed with the slit lamp microscope via a Canon digital camera.
2.7. PPE Trials
The PPE trials undertaken with high-speed bur included: (1) an N95 (FFP2 respirators New Zealand Government supplies) mask and goggles, (2) air-fed mask on 150l/min, (3) air-fed mask on 300l/min, (4) air-fed mask on 300l/min combined with an N-95 mask, and (5) the air-fed mask under plastic hoods with a low air consumption 20l/min. Makeshift hoods were created in two ways. One was a disposable plastic bag (bin liner), and the other a disposable plastic hooded raincoat (LDPE Rain Jacket LCR7042, China). A hole was cut in the plastic bag for the visor of the air-fed mask. The sticky tape was used to seal each hood around the visor. The hood was tucked under a hospital-issued long sleeve impermeable gown (Isolation Gown, Jackson Allison, New Zealand) (PPE gown). The raincoat provided a single-piece disposable hood and gown, so the hospital-issued PPE gown was not required, and the sticky tape was used to seal around the visor of the air-fed mask (Fig. 2).
3. RESULTS
For each trial of PPE, 3 tests were run in 3 different individuals. In the test of the N95 mask and goggles without the air-fed mask, the slit lamp examination revealed that there was considerable microscopic contamination of the lips and nasal hairs (Fig. 3). Eye contamination was prevented with the goggles worn very tightly, but at this level, without airflow, they fogged up with time. When the air-fed mask was run at 150l/min of air consumption, there was also considerable contamination of the facial skin under the mask and the mucous membranes of the eyes, nose, and lips. The performance of the air-fed mask improved markedly when run at its maximal setting of 300l/min compared to 150l/min. There was no skin contamination at this increased level, but micro-droplets could still be seen on nasal hairs. All facial contamination was eliminated when an N-95 mask and safety glasses were worn under the air-fed mask set at an air consumption rate of 300l/min. However, in all these tests, Wood’s lamp examination revealed contamination of the head and neck regions not covered by PPE.
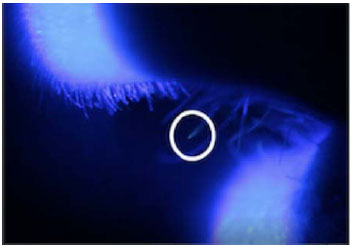
When the makeshift hoods (both the plastic bag and the disposable raincoat sealed around the visor) were used over the air-fed mask, the face and its mucous membranes were fully protected from any contamination. The hoods also prevented contamination of any part of the head and neck. We repeated the tests of the air-fed mask 3 times with the PPE gown and plastic bag hoods, then 3 times with the plastic raincoat; three different individuals were used in each set of PPE tests. In all these tests, the window around the hood was sealed to the visor with sticky tape, so the head and neck were physically sealed off. There was no contamination of the eyes, nose, or mouth under the slit lamp examination. There was also no contamination of the skin of the face, neck, or hair when examined with a Wood’s lamp.
Visual representation of results organised by PPE systems described in 2.6 PPE Trials.
4. DISCUSSION
In this investigation, we developed and tested a new personal protective equipment approach for use during the COVID-19 pandemic. It was found to be efficacious and inexpensive.
The objective of these study conditions was to test protective equipment beyond its normal clinical requirements. There was no ambient ventilation or high volume evacuation in the test conditions, and no attempt was made to mimic the confinement of the drill to an environment similar to the oral cavity. We consider this test to be more rigorous than the clinical situation.
The SARS-CoV-2 virus has been consistently detected in saliva, so there is a potential for spread during dental procedures [4]. Transmission may occur across the mucous membranes of the eyes, nose, or mouth and in the airway or lungs [4, 5]. As the droplet size in the aerosols decreases, droplet nuclei behaviour is less predictable, and protection becomes more difficult [6-8].
 |
||
| (1) |  |
Substantial contamination of the facial skin, eyes, lips, and nasal hairs. |
| (2) |  |
The use of goggles proved successful in preventing contamination of the eyes. They had to be worn so tight; however, they were uncomfortable for long periods and would gradually fog up with moisture from the eyes. |
| (3) |  |
Considerable contamination of the facial skin under the mask and mucous membranes of the eyes, nose, and lips. |
| (4) |  |
No facial skin contamination; however, micro-droplets could still be seen on nasal hairs. |
| (5) |  |
All facial contamination was eliminated. Head and neck regions were still exposed to the aerosol. |
| (6) |  |
Air-fed mask, sealed hood, low flow. No contamination of face, head, or neck was observed. |
Typically, dental enamel and hard dental materials are cut with burs driven by high-speed handpieces. The high-speed air dental drill (driven by an air turbine) was first designed in the late 1940s by New Zealand’s Sir John Walsh. Since then, handpieces have been evolved to typically operate in the range of 180,000 to 330,000rpm, with some running at up to 400,000rpm [6].
We first tested a hospital-issued N-95 mask. While N-95 masks are superior to surgical masks in protecting the wearer, they have an external leakage within the 3-5% range [7, 8]. In this study model, the N-95 mask did not protect the facial mucous membranes from micro-contamination by aerosol produced using the high-speed handpiece and diamond bur.
The air-fed mask used in this experiment is typical of devices used in the car body paint spraying industry. A foam diffuser inside the mask located under the chin discharges the air, allowing it to flow over the inside of the visor. Excess supplied air and exhaled air pass through or around an elasticated edge and into the ambient atmosphere. The manufacturer’s instruction for this mask is to run with an air consumption between 150-300l/min of air. Under our test conditions, the air-fed mask failed to provide good protection with an air-consumption rate of 150l/min with the high-speed handpiece. When increased to 300l/min, we still observed micro-contamination of the eyes, nose, and mouth, albeit less than what was observed with sole use of the N-95 mask. When the N-95 mask was used in combination with the air-fed mask set at a consumption rate of 300l/min, it prevented contamination. It appears that the microscopic droplets produced by high-speed drilling have such a small mass that they are not stopped by the airflow of the mask.
By adding the hood system, the droplet nuclei were no longer able to contaminate the head and neck, which meant that the air consumption rate of the mask could be greatly reduced. The system was more comfortable with less airflow as it did not make noise and made verbal communication easier. There was also less ballooning of the hood and less airflow down over the shoulders, which reduced the operator’s tendency to get too cold. A lower flow also meant that there was less burden on the compressor. Three masks run at 20l/min were within the capacity of a standard dental compressor for a 3-4 chair facility.
In our test, the goggles worn tightly prevented contamination of the eyes, but they were uncomfortable to wear and tended to fog up. The air-fed mask under the plastic hood provides comfortable, fog-free eye protection and allows enough space for loupes to be worn. A headlight can be used on loupes inside the mask or fixed externally.
Our combined set-up equipment of 3 air-fed masks, hosing, and filters cost $US 1043.00. The existing air compressor of the practice was used. The disposable costs of a plastic bag or disposable plastic raincoat are minimal.
As with all PPE, good hand hygiene and appropriate donning and doffing sequence must be maintained. Our recommended sequence is as follows: a window is made in the plastic bag and sealed with sticky tape before it is put on. The belt with the connections is put on. The mask and plastic bag are then placed together. Once the mask and hood are put on, a second person immediately connects the air and then helps tie the PPE gown at the back. The gloves are placed as for a normal surgical gown. When doffing, the reverse happens; a second person disconnects the tubing off the belt. The gown and gloves are then removed as one, inside out. The hood is turned inside out and lifted over the air-fed mask as it is removed. Once the mask is removed, the plastic bag is peeled off the mask and discarded. The visor area is then sanitized.
This study was initiated out of a need to protect dental workers when there was a shortage of PPE. Once PPE became available to us, we decided to test and compare our system with the hospital-issued PPE. A system of PPE was tested and developed to provide a very high level of protection in a demanding test model. In clinical practice, with the added protection of suction, room ventilation, and the relative confinement of the oral cavity, this novel PPE could be expected to provide excellent protection. To date, this system has been used in over 150 clinical cases without concerns. This idea could be further modified and tested into a more refined clinical product. Further comparisons of this novel PPE could also be made with other PPE such as positive air pressure respirators or other makeshift devices, like the modified snorkel mask and filter. Other dental surgery handpieces could be tested at different speeds to determine the threshold at which droplet nuclei become problematic.
CONCLUSION
A simple, low cost, and effective protection against dental aerosol using readily available industrial products is described and tested. Different PPE was tested in a shower cubicle with aerosol generated by dental handpieces. The water was dyed with fluorescein, and the study participants were examined under Wood’s lamp and a slit lamp with a cobalt blue light. Combining an air-fed paint spray mask on a low flow rate with a sealed hood prevented all head and neck contamination from a high-speed dental handpiece aerosol. This novel technique proved to have superior efficacy than our hospital-issued PPE in our test conditions. We believe our model will allow us to safely continue to provide urgent and emergency care of our dental patients during the SARS-CoV-2 pandemic.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
Not applicable
CONSENT FOR PUBLICATION
Informed consent was taken from all study participants.
AVAILABILITY OF DATA AND MATERIALS
The data sets used during the current study can be provided from the corresponding author [J.B.B] ,upon reasonable request.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
We would like to thank Mr. Matthew Swindlehurst from Medworx Biomedical Services for the technical set-up and Professor WM Thomson (University of Otago) for his advice on technical writing.


