A Pilot Study on the Degree of Tooth Staining Caused by Pollen Water and Chlorhexidine: In Vitro Study
Abstract
Background:
Chlorhexidine oral rinse has been used as an adjuvant in the treatment of periodontal disease. However, there are drawbacks of using chlorhexidine i.e. tooth staining and other side effects, including allergy reaction. In light of the proven therapeutic properties of pollen water as well as its relatively cheap cost in the market, pollen water has a potential to be an effective alternative to chlorhexidine oral rinse. The aim of this study is to compare the degree of tooth staining influenced by water-based pollen mouthwash to the standard Chlorhexidine mouthwash using spectrophotometer.
Materials and Methods:
24 specimens from extracted intact human teeth were soaked into the three different solutions, Chlorhexidine, Pollen water (date palm pollen water suspension), and normal water. Color measurements were carried out by a spectrophotometer devise and recorded at 5 different time intervals. Color change (∆E), Chroma (C*) and Hue (H*) were analyzed and compared among the three solutions.
Results:
Overall mean ∆E was similar in all groups, significant difference between all time points was found only in pollen water. The change in C* was higher in pollen water as compared to other solutions. There was a subtle increase in H* in the Chlorhexidine samples after week 3. The H* values in pollen water were stable, but a sudden decrease was observed in week 6. The difference in H* among the three solutions was significant after 3 weeks.
Conclusion:
Within the limitation of our study, it can be concluded that Pollen water stained teeth to a lesser extent than did chlorhexidine. It might be beneficial to use Pollen water as mouthwash however, further investigation is needed regarding the efficacy of plaque control.
1. INTRODUCTION
Natural products have been used for medicinal purposes throughout human history for their anti-inflammatory and antimicrobial properties, among others [1-4]. Several clinical studies have reported the therapeutic oral effects of various natural products [1, 2]. Moreover, pollen water is cultivated in the Kingdom of Saudi Arabia, especially in the eastern province. Traditionally, pollen water has been used to treat various conditions, including gastrointestinal and immune problems, in addition to being used as an oral rinse for improving overall health [4].
Periodontal disease is a common inflammatory disease affecting all ethnicities [5]. Proper oral hygiene is essential for preventing periodontal disease and dental caries, and optimal wound healing after periodontal surgery (e.g., implant surgery and periodontal plastic surgery) requires keeping the surgical area as inflammation-free as possible [6-8]. In these regards, oral rinses with anti-plaque and anti-inflammatory properties could be useful [8].
Chlorhexidine oral rinse is a mouthwash used as an adjuvant in periodontal disease treatment; however, it has several disadvantages [9]. Previous studies have reported it bears a risk of severe allergic reactions, including anaphylaxis; moreover, several patients have reported local short-term reactions such as mucosal irritation, as well as other side effects, including tooth staining [9].
The recommended Chlorhexidine usage time is approximately one to two weeks, with extended usage increasing its drawbacks and reducing its benefits [7, 10].
During the healing process after periodontal surgery, including implant surgical procedures and periodontal plastic surgery, the surgical area should be kept as inflammation-free as possible to allow optimal wound healing [6-8]. For this purpose, chlorhexidine is normally prescribed for the patients to obtain dental plaque free for the surgical area since mechanical irritation by regular brushing should be avoided [9]. Given the current increase in microbial resistance to antimicrobials and the side effects of synthetic chemicals, there is a demand for natural products [11]. Based on the proven therapeutic properties of pollen water, as well as its relative cheapness, it could be an ideal candidate for use as an oral health product [4].
Spectrophotometer is one of the most accurate, useful, and flexible measurement devices for overall color matching in dentistry [12]. It measures the light energy reflected from an object at 1–25 nm intervals along the visible spectrum [13, 14]. It contains an optical radiation source, a means for dispersing light, an optical measurement system, a detector, and a means for converting light obtained to an analyzable signal. Measurements obtained by these instruments are frequently keyed to dental shade guides and converted to shade tab equivalent [15]. Compared with observations by the human eye or conventional techniques, spectrophotometers have been reported to offer an accuracy increased by 33% and a more objective match in 93.3% of cases [16].
Pollen water could be an effective alternative to chlorhexidine. There have been no studies on pollen water and its effect on tooth staining. In this pilot study, we aimed to use spectrophotometer device to measure and compare the degree of tooth staining caused by pollen water-based mouthwash to that by standard Chlorhexidine mouthwash. This is the first study to investigate the effect of pollen water on color change/tooth staining. The null hypothesis is that there is no difference in teeth staining or discoloration when teeth are exposed to pollen water-based mouthwash compared to teeth exposed to Chlorhexidine mouthwash.
2. MATERIALS AND METHODS
This study was conducted at the laboratory of Imam Abdurahman bin Faisal University College of Dentistry. It consisted of the following steps:
(1) Preparation of three solutions:
● Chlorhexidine 0.12% licensed for use as a mouthwash. (positive control)
● Pollen water (date palm pollen water suspension) obtained commercially from a manufacturer (Pollen Water; Liwa Retail Products, Dubai, United Arab Emirates), licensed for internal/external use (100% pure, cold-pressed). (test group)
● Distilled water. (negative control)
(2) Teeth preparation: 24 extracted intact human molar teeth were used in this study. Inclusion criteria were: caries-free teeth with no cracks, no previous root canal therapy, and no crowns, which were recently extracted for orthodontic treatment purposes. The teeth surfaces were cleaned using a scaler to dislodge debris and periodontal instruments to remove calculus. This was followed by brushing using a rubber cup. Subsequently, each tooth was decoronated, and the crown was prepared to be at a size of 8 mm ×11 mm × 2.5 mm using a diamond bur (Iwanson, Ustomed, Tuttlingen, Germany). The total sample size was 24 (8 samples for each solution).
(3) Color Measurements: Each prepared sample was placed onto the chamber of a benchtop digital imaging spectrophotometer (X-rite color-Eye 7000A; Gmbh, Regensdorf, Switzerland), which was previously calibrated according to the manufacturer’s specifications. Color measurements were obtained in triplicate, and the mean value was considered as the final measurement.
(4) Each specimen was soaked in Chlorhexidine, pollen water, or normal water, taking in mind changing the solutions every day, and staining was measured weekly for 6 weeks.
(5) The following parameters were analyzed:
● ∆E: indicates the change in color from baseline to that at the measurement time.
● Chroma (C*): indicates the color intensity or saturation
● Hue (H*): describes the perception of an object’s color (e.g., red, orange, green, blue).
2.1. Statistical Analysis
The color change (∆E) was calculated using the following Eq. (1):
Where:
∆ represents the difference between the object being measured and its reference
L* represents lightness
a* and b* are the chromaticity coordinates
Data normality was checked using the Shapiro-Wilk test with p < 0.05, indicating non-normally distributed data. The color differences for L, a, b, C*, and H* were calculated and analyzed using the Friedman test (non-parametric method of repeated-measure analysis of variance (ANOVA)). Repeated-measure ANOVA was applied separately for each solution. For significant findings, the paired sample t-test was performed to assess for individual significant results. We considered it statistically significant at P < 0.05. All analysis was carried out using SPSS version 22 software (IBM, Armonk, NY).
3. RESULTS
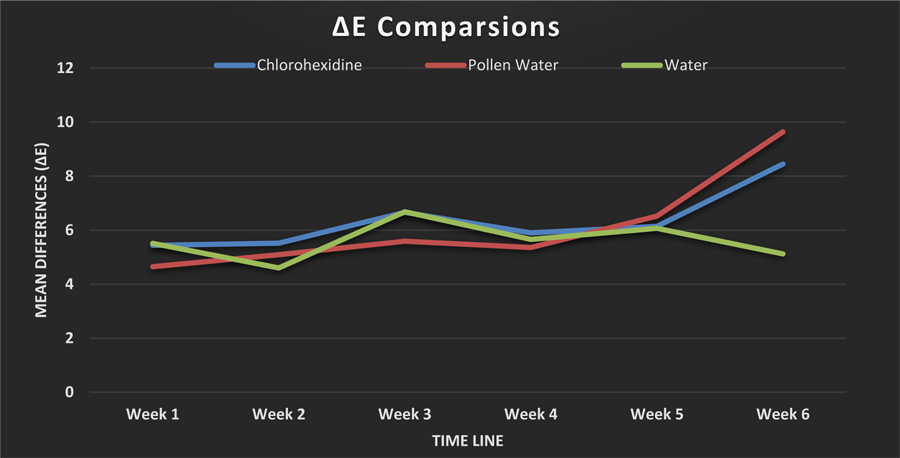
Table 1 presents the mean ∆E values (color changes relative to baseline) for the tooth samples after their submersion in pollen water, Chlorhexidine (positive control), or water (negative control) for 1 to 6 weeks. The overall mean ∆E differed significantly over time in the pollen water group (p = 0.017) but not in the Chlorhexidine or water group. Paired t-tests in the pollen water group showed significant changes in ∆E between weeks 1 and 5 (p = 0.036) and weeks 1 and 6 (p = 0.012). There was no significant change between weeks 2 and 4 in this group, nor were there significant differences in the overall ∆E values among the solutions at any time. ∆E increased with time in both the Chlorhexidine and pollen water groups but did not change after 1 month in the water group (Fig. 1).
As shown in Table 2, there were no significant changes in color saturation (C*) at any time in any group. In the pollen water group, C* decreased dramatically in week 3 and increased dramatically in week 6. Compared with the other groups, the changes in C* were most pronounced in the pollen water group.
| Solution | ∆E Week 1 |
∆E Week 2 |
∆E Week 3 |
∆E Week 4 |
∆E Week 5 |
∆E Week 6 |
p-value |
|---|---|---|---|---|---|---|---|
| Chlor-hexidine | 5.44 ± 2.8 | 5.52 ± 3.12 | 6.66 ± 3.3 | 5.9 ± 2.41 | 6.14 ± 3.4 | 8.45 ± 4.93 | 0.343 |
| Pollen water | 4.65 ± 1.73a | 5.09 ± 1.82b | 5.59 ± 2.16 | 5.36 ± 1.84c | 6.52 ± 2.29a, b,c | 9.64 ± 5a | 0.017 |
| Water | 5.51 ± 2.57 | 4.6 ± 2.46 | 6.68 ± 2.95 | 5.66 ± 2.77 | 6.07 ± 2.3 | 5.12 ± 2.91 | 0.273 |
| p-value | 0.325 | 0.135 | 0.687 | 0.607 | 0.100 | 0.325 |
| Solution | ∆C Week 1 |
∆C Week 2 |
∆C Week 3 |
∆C Week 4 |
∆C Week 5 |
∆C Week 6 |
p-value |
|---|---|---|---|---|---|---|---|
| Chlor-hexidine | 0.17 ± 1.03 | 0.26 ± 1.53 | 0.7 ± 1.82 | 0.45 ± 1.5 | 0.52 ± 1.74 | 0.1 ± 1.57 | 0.755 |
| Pollen water | 0.62 ± 1.28 | 0.68 ± 1.46 | 0.04 ± 0.72 | 0.59 ± 1.19 | 0.22 ± 1.04 | 2.32 ± 3.23 | 0.061 |
| Water | 0.53 ± 0.86 | 0.96 ± 1.09 | 0.08 ± 1.2 | 0.02 ± 1.11 | 0.17 ± 1.03 | 0.78 ± 1.54 | 0.090 |
| p-value | 0.325 | 0.417 | 0.882 | 0.882 | 0.88 | 0.417 |
| Solution | ∆H Week 1 |
∆H Week 2 |
∆H Week 3 |
∆H Week 4 |
∆H Week 5 |
∆H Week 6 |
p-value |
|---|---|---|---|---|---|---|---|
| Chlor-hexidine | 5.77 ± 8.65 | 17.02 ± 21.26 | 15.54 ± 34.62 | 23.81 ± 24.77 | 27 ± 29.49 | 29.14 ± 29.11 | 0.004 |
| Pollen water | 2.75 ± 0.87 | 3.55 ± 2.12 | 3.66 ± 1.95 | 3.42 ± 1.86 | 3.91 ± 1.75 | -0.57 ± 5.89 | 0.243 |
| Water | 2.4 ± 0.84a | 3.09 ± 0.99 | 2.95 ± 1.87 | 4.4 ± 2.13 | 0.28 ± 13.75b | 2.49 ± 3.54 | 0.027 |
| p-value | 0.197 | 0.135 | 0.417 | 0.021 | 0.03 | 0.01 |
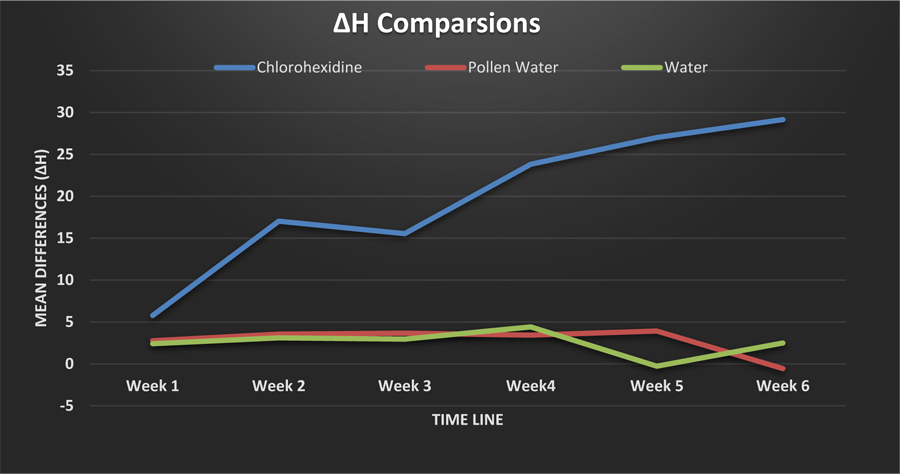
As shown in Table 3, there was a subtle increase in the hue indicator (H*) in the Chlorhexidine group, most noticeably after week 3. Contrastingly, in the pollen water group, H* was stable throughout the weeks, however, a slight decrease was noticed in week 6 (Fig. 2). H* differed significantly among the three groups after 3 weeks of sample submersion.
4. DISCUSSION
Tooth staining impedes esthetic and restorative treatment. Currently, there is a huge demand for esthetic considerations in optimal teeth color matching as part of a beautiful smile across age ranges. Continuous exposure of teeth to various oral environmental factors, including water, hot and cold drinks, and mouth rinses, can affect them physically and appearance-wise. Therefore, evaluating their color stability and staining resistance is crucial.
Our study evaluated and compared the color stability of dental enamel exposed to three different mouthwash solutions, i.e., Chlorhexidine (positive control), pollen water (test group), and normal water (negative control). The color change was measured using a benchtop digital imaging spectrophotometer (X-rite color –Eye 7000A spectrophotometer, Gmbh, Regensdorf, Switzerland) that uses pulsed xenon illumination with a spectral range of 360-750 nm [17]. This device combines the benefits of a traditional spectrophotometer with digital photography, allowing a more accurate color change evaluation than that of traditional spectrophotometers [18].
Surrounding light could play an important role in the clinical assessment of teeth color [19]. To ensure that ambient light did not distort tooth color during spectrophotometric measurement, constant light conditions were standardized.
In our study, the change in H* for teeth submerged in Chlorhexidine was the most pronounced change. Several previous studies [9, 20] have reported severe color change associated with Chlorhexidine mouthwash. However, this effect has been reported as highly dependent on the trial duration [21]. An in vivo study by Bagis et al. [22], who evaluated tooth staining effect of Chlorhexidine mouthwash on 24 participants, reported that its effect on natural dentition was the greatest on the first three days of use.
In our study, there was no significant difference in ∆E over time in the Chlorhexidine group. This is consistent with the findings of Moreira et al. [23], where they evaluated color changes of bovine teeth after prolonged exposure to different mouth rinses. In that study, discoloration in the Chlorhexidine group was clinically imperceptible. Chlorhexidine staining mechanism of action was previously explained, as it is due to the precipitation of food pigments on dental surfaces after rinsing [24]. Because there was no food used in their study, as well as in our study, this might justify why the discoloration in the Chlorhexidine group was not clinically perceptible.
Spectrophotometers have been reported to allow a 33% increase in accuracy compared with observations by the human eye. Moreover, they allow a more objective color match in 93.3% of cases [16].In our study, a captured image was taken from standardized evaluation area (8 mm × 11 mm) of each sample, where the color was analyzed using the following Commission Internationale de lÉclairage color coordinates: L* (lightness), a* (green-red coordinate), and b* (blue-yellow coordinate). Using these coordinates, termed the CIELAB color coordinates, the color change can be calculated as ∆E. When the material tested is color stable, there is no color difference after exposure to the testing environment (∆E * = 0). In the CIE lab color system, ∆E values > 3.3 units are considered clinically detectable [25]. In the current study, all ∆E values were >3.3, which indicates that the color of the solution at a given time point can be physically differentiated from its color at baseline.
We used the spectrophotometer device (X-rite color –Eye 7000A spectrophotometer, Gmbh, Regensdorf, Switzerland) to calculate the CIE2000 ∆L’ (differences in lightness), ∆C’ (differences in C*), and ∆H’ (differences in H*). H* can be described as the perception of an object’s color, e.g., red, orange, green, blue. The opponent-colors theory of color is applicable where color cannot be simultaneously green and red or blue and yellow [26]. Therefore, single values can be used to describe the red/green and yellow/blue scales. On the other hand, C* defines the color intensity or saturation. We measured differences in C* (∆C’) and H* (∆H’), which can be expressed as positive or negative values. Positive ∆C’ values indicate a brighter or more intense color, whereas negative ∆C’ values indicate a dull or less intense color. Positive ∆H’ values indicate a more reddish/yellowish color, while the negative ∆H’ values indicate a greenish/bluish color.
There was no significant change in C* measurement at any stage for any solution in our study. There was a subtle increase in H* after week 3 in the Chlorhexidine-treated samples. This is consistent with previous studies showing that the major drawback of Chlorhexidine mouth rinse is yellow-brown tooth staining [23]. H* values in the pollen water group were relatively stable, excepting a sudden decrease in week 6. This decline may be particularly clinically important because pollen water-based mouth rinses can discolor teeth along the green/blue scale. However, longer immersion times are required to confirm our results.
CONCLUSION
To our knowledge, this is the first study that evaluated the effect of pollen water-based mouthwash on tooth staining, and compared it to the Chlorhexidine staining effect. Pollen water showed promising results in terms of Hue differences compared to other groups. These findings suggest that pollen water has less impact on tooth staining than does Chlorhexidine. However, the influence of long-term aging, with larger sample size is yet to be investigated. In conclusion, and within the limitation of this study, the use of pollen water as an oral rinse could have benefits over Chlorhexidine; however, there is a need for further studies to assess its efficacy in plaque control.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was conducted at the laboratory of Imam Abdurahman bin Faisal University College of Dentistry, after gaining ethical approval (EA #2018029) from the Scientific Research Unit.
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures were followed in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article is in the [Dryad], available from: [https://datadryad.org/stash/share/ KFNPunv6G7cLrHJrCWLIAwfe9sRJKV_YcPoxWL8ykCI].
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors wish to thank Ms. Miare Kuse for her assistance.


