Rehabilitation of Atrophic Posterior Mandible with Vertical Ridge Augmentation with Resorbable Membrane: Case Report with 3-Year Follow-up
Abstract
Background:
The placement of implants in the posterior mandible with the vertical bone defect can be associated with inadequate crown height space. Therefore, the vertical bone reconstruction of these defects is often necessary, although this procedure is technically challenging.
Methods:
A 49-year-old patient attended a private dental office for rehabilitation of an atrophic posterior mandible. The clinical and tomographic findings show absence of teeth #36, #37, #46, #47, and #48 with severe atrophy. Vertical bone augmentation was performed by using the guided bone regeneration technique with pericardium resorbable membrane followed by placement of short implants. The free gingival graft was performed, and after three months, screw-retained lithium disilicate single ceramic crowns were manufactured.
Results:
After a 3-years follow-up, bone loss around the implants or presence of gingival inflammation was not observed, and the prosthesis adaptation was found to have no alteration either. Therefore, aesthetics, as well as masticatory and speech functions, were preserved.
Conclusion:
There was no bone loss around the implants. The association between vertically guided bone regeneration using pericardium resorbable membrane is an alternative technique, and it avoids complications related to non-resorbable membrane exposure. It was shown to be viable after a 3-year follow-up.
1. INTRODUCTION
The mandibular posterior region frequently shows three-dimensional bone defects, demonstrating limitations during its rehabilitation mainly due to the presence of noble structures, such as the mandibular canal and inferior alveolar nerve. In addition, there is often a deficiency of the soft tissue in terms of quantity and quality in this region [1]. In this way, surgical techniques were developed to enable the placement of implants in the mandibular posterior region [2, 3]. Among all the techniques available, guided bone regeneration (GBR) is currently the most used approach for bone reconstruction in implant dentistry [4].
The membranes can be rated as resorbable [4] and non-resorbable [4, 5], and they have specific characteristics to be used in the GBR [4]. In three-dimensional bone defects, there is no bony wall, that is, without enough bone tissue to support and stabilise the reconstructive material and clot. For this reason, bone healing can become compromised since angiogenesis must reach a certain distance from native bone for the bone substitute to allow re-vascularisation and formation of the new bone [6]. Moreover, the soft tissue must be well detached and released to prevent strain in the region during the suture, thus facilitating the primary intention of healing [6]. Therefore, the reconstruction of vertical bone defects in the posterior region of the mandible is considered one of the most complex defects to perform the GBR technique.
In this context, GBR is a more predictable and favourable procedure for three-dimensional reconstructive procedures in the posterior region of the mandible, especially when associated with non-resorbable membrane and placement of short implants [5]. However, the non-resorbable membrane has disadvantages and complications, such as the removal of the membrane and its exposure [4, 5].
In this way, the present clinical case describes the first clinical case showing an approach for rehabilitation of atrophic posterior mandible in which vertical ridge augmentation was performed with resorbable membrane instead of non-resorbable devices, thus enabling the placement of implants and CAD/CAM prosthesis.
2. MATERIALS AND METHODS
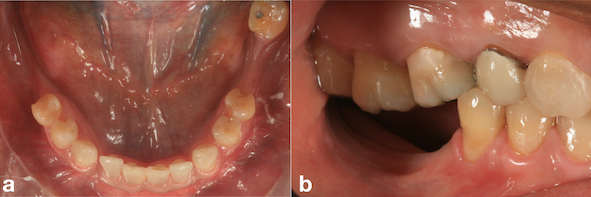
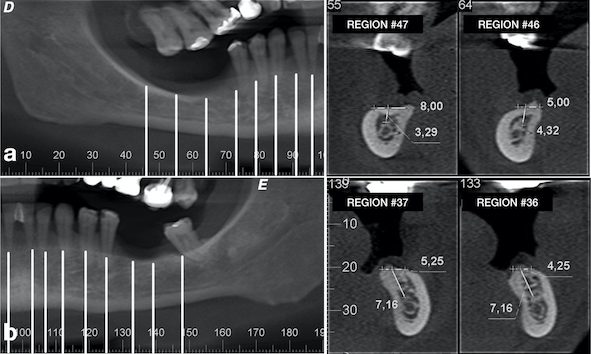
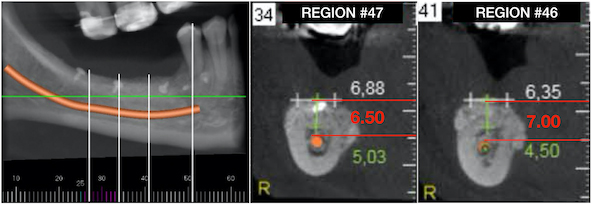
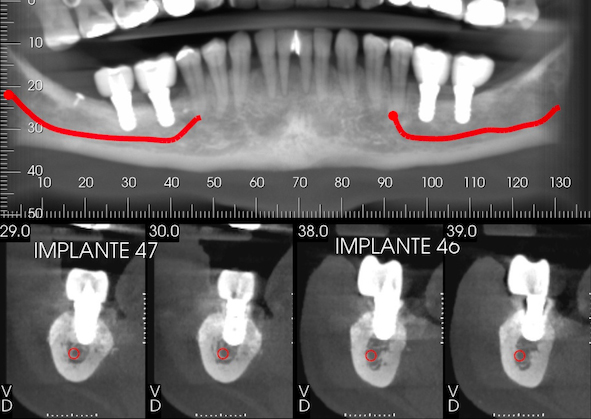
A 49-year-old patient attended a private dental office in the city of Fortaleza, CE, Brazil, for rehabilitation of an atrophic posterior mandible. During anamnesis, no systemic disease was reported. Clinical evaluation showed absence of teeth #36, #37, #46, #47 and #48 and significant bone resorption in these regions (Fig. 1a), including extrusion of teeth #16, #17 and #18 (Fig. 1b). Cone-beam computed tomography (CBCT) showed that the patient had a limited bone height in the region of teeth #46 and #47 with a distance of 3-4 mm between the bone crest and mandibular canal thus hindering the placement of implants (Fig. 2a). With regard to the left lower region, there was enough bone for the placement of 6-mm implants in the region of teeth #36 and #37, as well as the presence of Grade-III furcation defect and alveolar bone loss in the region of tooth #38 (Fig. 2b). The case report was approved by the Research Ethics Committee of the University of Santo Amaro. Before the treatment, the informed consent form was presented and signed by the patient.
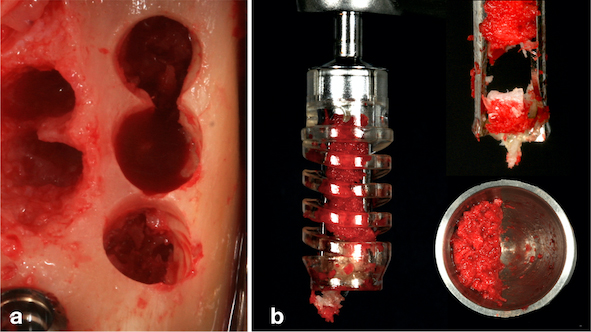
Under local anaesthetic infiltration with articaine hydrochloride 4% with epinephrine 1:200,000 (Nova DFL®, Rio de Janeiro, RJ, Brazil), a crestal mucoperiosteal incision was made at the intra-sulcular region of tooth #35 and distally extended to the distal region of tooth #38, thus exposing the mandibular ramus. Curettage of the alveolus and granulation tissue was performed after extraction of tooth #38. Subsequently, two implants measuring 4.1 x 6.0 mm (Standard Plus SLActive, Roxolid®, Straumann, Basel, Switzerland) were placed in the region of teeth #36 and #37 according to the manufacturer’s instructions, with a final insertion torque of 35 N.cm. Next, healing caps were placed. Removal of the particulate autogenous bone graft in the region of the mandibular ramus was performed with a specific drill at low speed (300 rpm) with enough irrigation, according to the manufacturer’s instructions (ACM, Neobiotech, Seoul, South Korean) (Fig. 3a and b). The flap was closed with a non-absorbable suture (5-0 Resolon®RESORBA® Medical GmbH, Nüremberg, Germany). The removed autogenous bone graft was associated and mixed with the osteoconductive biomaterial (Cerabone®, Botiss, Dieburg, Germany) at a ratio of 1:1, according to the technique proposed by Urban et al., 2017 [6].
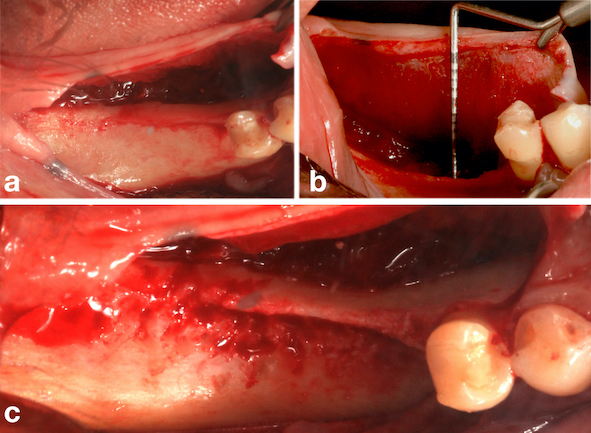
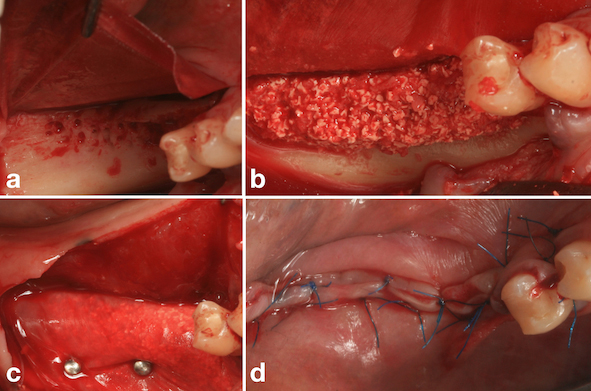
During the same operative procedure, an intrasulcular mucoperiosteal incision was made in the region of tooth #45 and distally extended to the crestal region of tooth #48, followed by an oblique incision distal to the bone defect. A vertical incision of 3-4 mm was made in the mesio-lingual region of tooth #45 to facilitate approximation of the tissues and passivity of the suture following the reconstructive procedure (Fig. 4a). After large displacement of the buccal and lingual flaps (Fig. 4b), decortication of the bone defect was performed (Fig. 4c). Three bone tacks (Micro plant, São Paulo, SP, Brazil) were used in the lingual region of the bone defect for fixation of the porcine pericardium collagen membrane (Jason Membrane®, Botiss, Dieburg, Germany) (Fig. 5a), followed by placement of autogenous bone graft associated with osteoconductive biomaterial (Cerabone®, Botiss, Dieburg, Germany) (Fig. 5b). The final fixation of the membrane was performed by using three bone tacks (Micro plant, São Paulo, SP, Brazil) in the buccal region (Fig. 5c). Finally, closure was performed with non-resorbable sutures (5-0 Resolon®. RESORBA® Medical GmbH, Nüremberg, Germany) (Fig. 5d). The post-operative medication consisted of antibiotics (875 mg amoxicillin + 125 mg clavulanate every 12 hours for 7 days) and anti-inflammatories (400 mg ibuprofen every 6 hours for three days), including a soft diet.
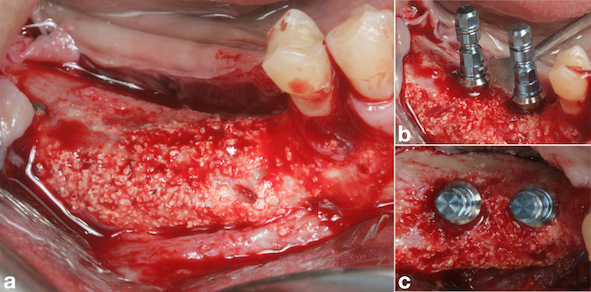
After one month from the surgical procedure, a T-plate was placed (Neodent®, Curitiba, PR, Brazil) in the buccal region and also a mini-implant (Neodent®, Curitiba, PR, Brazil) in the palatal region between teeth #17 and #18 (Fig. 6a). Moreover, two brackets (Morelli, Sorocaba, SP, Brazil) were also bonded to these teeth for activation of the plate and mini-implant in order to intrude them, thus creating space for placement of the prostheses (Fig. 6b). After six months, another CBCT was performed to assess the vertical and horizontal reconstruction. There was vertical bone augmentation in the region of teeth #46 and #47, respectively, by 2.68 mm and 3.21 mm (Fig. 7), which allowed the placement of 6-mm implants. The same medication and anaesthetic protocol used in previous surgery were used for this surgical step. A mucoperiosteal incision in the alveolar crest was performed (Fig. 8a), followed by a detachment of the flap and placement of two implants of 4.1 x 6.0 mm (Standard Plus SLActive, Roxolid®, Straumann, Basel, Switzerland) according to the manufacturer’s instructions (Fig. 8b) and (Fig. 8 and c). Post-operative medication and guidance were the same used after placement of implants on the contralateral side.
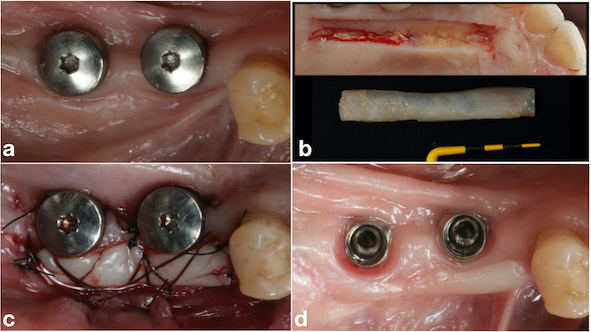
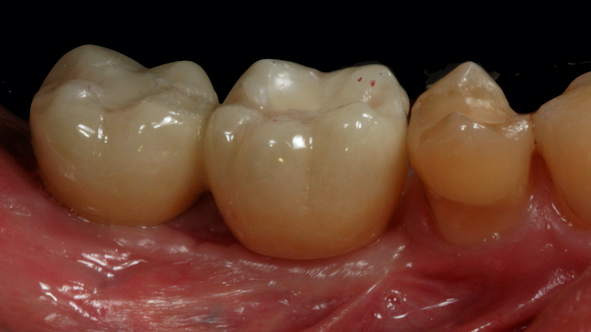
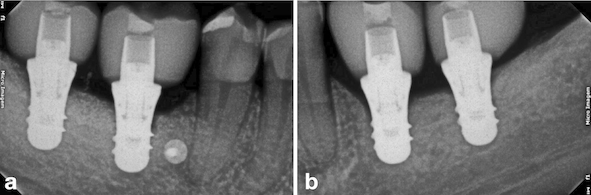
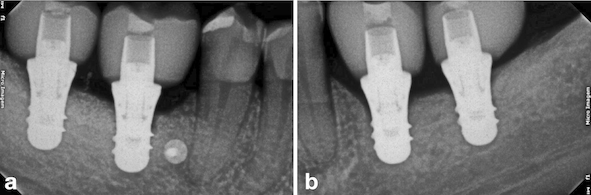
After two months from the placement of the implants in the region of teeth #46 and #47 (Fig. 9a), the free gingival graft was removed from the palatal region of teeth 23# and 27# (Fig. 9b). The grafts were fixed by crossed horizontal sling suture and interrupted sutures with 5-0 Nylon wire (Ethicon®, Jonhson& Johnson, São José dos Campos, São Paulo - Brazil) (Fig. 9c). Screw-retained lithium disilicate single ceramic crowns were manufactured after three months (Fig. 9d) by using the CEREC CAD-CAM system, and it was cemented onto a Variobase-C® (Straumann, Basel, Switzerland) abutment with a final torque of 35 N.cm (Figs. 10 and 11). After a 3-years follow-up, bone loss around the implants or presence of gingival inflammation was not observed, and the prosthesis adaptation was found to have no alteration either (Fig. 12). Therefore, aesthetics, as well as masticatory and speech functions were preserved.
3. DISCUSSION
The first technique to be used for the vertical reconstruction was onlay autogenous grafts because of their properties of osteogenesis, osteoinduction, and osteoconduction [7]. However, the results reported in the literature show that bone graft resorption can occur over time and even following the placement of the implants [7].
Urban et al., 2019 [8] conducted a systematic review and a meta-analysis on the effectiveness of the techniques used to increase the vertical bone and found that osteogenic distraction is a procedure allowing more vertical gain but with a higher rate of complications. With regard to GBR, this is the most used technique for vertical ridge augmentation due to its superiority in terms of bone gain compared to onlay block graft, in addition to having the advantage of not using a second surgical site, which reduces complications [8].
Resorbable membranes show better results in comparison to non-resorbable ones regarding compatibility with soft tissues and lower rates of exposure and complications [4]. However, due to the chemical cross-linking process of different types of membranes, interference in their enzymatic degradation may occur, leading to prolonged biodegradation [9]. This can affect the GBR, mainly when vertical bone defects are involved since a prolonged degradation time of the membrane is needed so that the bone substitute can undergo angiogenesis and initiate the bone tissue neoformation.
In this clinical case, the GBR technique with pericardium membrane and ABBM mixed with autogenous particulate bone at 1:1 was used to increase the vertical bone sufficiently for placement of a short implant since the patient had a remaining bone height of 3-4 mm in the region of teeth #46 and #47 and because the bone defect was missing four bone walls. It was in the posterior region of the mandible, which is generally poorly vascularized. The resorbable membrane was chosen to avoid complications, which might occur with non-resorbable ones, such as exposure of the membrane, and because there was no need for a greater bone augmentation since the patient had a residual bone of 3-4 mm in the region. Therefore, two 6-mm implants were placed on the site. The bone increase achieved was 2.63 mm and 3.18 mm. This result is in accordance with a systematic review and meta-analysis performed by Urban et al. [8], who reported a vertical bone gain of 3.51 mm by using the GBR technique and the use of a resorbable collagen membrane.
One of the principles for the GBR predictability is the initial stability of the clot [6]. This property in the treatment of vertical bone defects becomes rather more difficult when using resorbable membranes than non-resorbable ones because of the likelihood of displacement of the collagen membrane. However, this is decisive for a successful reconstruction [6]. The stability of the membrane can be achieved based on its elastic properties. In this way, it is possible to accommodate the bone substitute in the recipient site and perform fixation of the membrane so that the reconstruction remains stable [6]. In this clinical case, this property was achieved due to the type of membrane used, which was a porcine pericardium one. In fact, this membrane is highly resistant to rupture and has a high degree of biocompatibility and prolonged resorption time due to the collagen structure in multiple layers, which in turn helps in the angiogenesis process [9, 10].
Considering the limitations of vertical bone gain up to 5 mm, the pericardium membrane is a favorable option in terms of minor surgical complications, alternative technique, and low-cost procedure when compared with non-resorbable membrane.
CONCLUSION
The GBR technique is considered predictable, but when performed in the posterior region of the mandible, there are some difficulties regarding the choice of an adequate membrane for stabilisation of the biomaterial as well as the quality of the recipient site, which may also be poorly vascularised. Thus, the GBR technique with pericardium membrane and ABBM mixed with autogenous particulate bone at 1:1 allowed the vertical ridge augmentations and the placement of short implants, which were associated with soft tissue reconstruction. These techniques allowed to achieve success in the treatment after a 3-years follow-up.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Ethical approval was obtained from the Ethics Committee of Universidade de Santo Amaro – UNISA, Brazil with approval no. 5502413.1.0000.0081.
HUMAN AND ANIMAL RIGHTS
No Animals were used in this research. All human research procedures were followed in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Not applicable.
STANDARDS OF REPORTING
CARE guidelines and methodology were followed.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


