All published articles of this journal are available on ScienceDirect.
Increased VEGF-A Expression of Human Dental Pulp Stem Cells (hDPSCs) Cultured with Advanced Platelet Rich Fibrin (A-PRF)
Abstract
Background:
VEGF-A expression of human dental pulp stem cells (hDPSCs) can induce the angiogenesis process of dental pulp regeneration. This in vitro study aimed to analyze the effect of various concentrations of Advanced Platelet Rich Fibrin (A-PRF) conditioned media (CM) on the increased expression of vascular endothelial growth factor-A (VEGF-A) of hDPSCs.
Methods:
hDPSCs were collected from ten third molars extracted from nine healthy donors, cultured, and then harvested at the end of the third passage. The hDPSCs were seeded in four different CM (control group: hDPSCs + DMEM; 1% A-PRF CM group: hDPSCs + 1% A-PRF CM; 5% A-PRF CM group: hDPSCs + 5% A-PRF CM; 10% A-PRF CM group: hDPSCs + 10% A-PRF CM). All of the groups were cultured in biological triplicates (Triplo) and observed for 5, 12, and 24 hours. The VEGF-A protein expression of hDPSCs was measured using human VEGF-A ELISA at a wavelength of 405 nm. Data was analyzed with Kruskal Wallis and post hoc Mann Whitney test with p<0.05.
Results:
The VEGF-A expression rate of hDPSCs among all groups was statistically significantly different at 5, 12 and 24 hours of observations (p<0.05). Post hoc analysis test showed a statistically significant difference of hDPSCs’s VEGF-A expression between 5% A-PRF groups compared to other groups at 5 and 12 hours of observation (p<0.05). However, there were no statistically significant differences observed of hDPSCs’ VEGF-A expression at 24 hours of observation between 1%, 5% and 10% A-PRF groups (p>0.05).
Conclusion:
5% A-PRF CM was superior in increasing VEGF-A expression of hDPSCs at 5, 12 and 24 hours of observations.
1. INTRODUCTION
American Association of Endodontists (AAE) approved regenerative endodontics as a potential future therapy in endodontics. Protocol in this type of therapy includes cell homing, migration and attracting factors for proliferation and differentiation of endogenous human Dental Pulp Stem Cells (hDPCs) [1-3]. Therefore, finding the suitable conditioned media (CM) for hDPSCs is one of the important factors that, for hDPSCs recruitment and growth, might provide the favorable niche environment for dental pulp regeneration [1, 3, 4]. Human dental pulp stem cells (hDPSCs) are mesenchymal stem cells (MSCs) or progenitor cells derived from dental pulp cells [5, 6]. HDPSCs have multilineage differentiation ability and can be differentiated into a number of different cells types, such as odontoblasts, osteoblasts, and endothelial cells [1]. The International Society for Cellular Therapy (ISCT) stated that 95% of human MSCs can express at least three types of specific surface antigens, CD105, CD73, and CD90/Thy-1 (positive cocktail) [7, 8].
The hDPSCs can express some endothelial cell markers, including CD31, CD105 and CD34 in vitro, depending on the modification of the culture medium [8, 9]. Bronckaers et al. [10] revealed that hDPSCs can express a number of angiogenesis protein molecules of blood-derived Growth Factor (GF), such as Vascular Endothelial Growth Factor (VEGF), Fibroblast Growth Factor-2 (FGF-2), and Monocyte Chemotactic Protein-1 (MCP-1) [10]. Moreover, VEGF increases the paracrine induction of hDPSCs in angiogenesis as a key process in dental pulp tissue regeneration and longevity [10]. Gharaei et al. [11] demonstrated that human dental pulp stromal cell conditioned medium can trigger pronounced angiogenic effects in increasing VEGF expression [11]. These results are consistent with that of another study proving that VEGF expression plays an important role in the endothelial differentiation of MSCs derived from human exfoliated deciduous teeth [12]. Tran-Hung et al. [13] showed that human dental cells can express Platelet Derived Growth Factor-AB (PDGF-AB), VEGF, and FGF-2 shortly (5 hours) after injury; the expression levels then start to peak in 24 hours [13].
Most in vitro studies have used fetal bovine serum (FBS) as a cell culture supplement to isolate and expand MSCs [14-16]. However, FBS are xenogeneic and may induce allergic responses; furthermore, the long-term culture of DPSCs with a high level of serum may lead to the spontaneous differentiation of cells [14-16]. The FBS itself can have a secretome that also can release a number of Growth Factors (GF) that can create a paracrine effect for cells [14]. Recently, the replacement of FBS as a culture medium for MSCs has been widely studied [15-17]. Asrianti et al. [17] revealed the use of human platelet lysate (hPL) as an alternative supplemented conditioned media (CM) for hDPSCs [17].
Research on the use of platelet products such as platelet-rich plasma (PRP), platelet-rich fibrin (PRF), and their modifications, for supplementing CM in regenerative endodontics, has majorly increased [18-24]. These platelet products can release growth factors (GFs), such as VEGF, FGF-2, Platelet Derived Growth Factor (PDGF) and cytokines that are needed in the dental pulp regeneration process [18]. One of the platelet CM modifications, advanced platelet-rich fibrin (A-PRF), can exhibit better GFs compared to their first-generation products (PRF) [18-20]. Kobayashi et al. [20] demonstrated that A-PRF releases higher quantities of GFs, such as Transforming Growth Factor-β (TGF-β), PDGF, VEGF, and CCL-5, compared to traditional PRF [20]. Moreover, A-PRF can also contribute to the induction of neutrophils [21]. Another study revealed that hDPSCs can reach the highest proliferation rates after being cultured in 25% A-PRF CM for 3-5 days [22].
Saaed et al. also demonstrated that 10% PRF exudate (PRF-Ex) can replace FBS as CM for hDPSCs and increase their differentiation ability into osteogenic cells [23]. The potential of A-PRF as a culture medium for hDPSCs has also been reported by Bagio et al. [24], who showed that 1%–5% A-PRF CM induces the dentin sialophosphoprotein (DSPP) expression of hDPSCs; furthermore, Alizarin red results showed the osteogenic differentiation of hDPSCs after 7 days of culture [24]. These results correlate with the osseointegration relationship in the ability of human MSCs (hMSCs) to express VEGF that exerts signaling with osteoblast differentiation [25]. Leukocyte PRF (L-PRF) CM can achieve a higher level of VEGF and Interleukin-8 (IL-8) expression compared with L-PRF-Ex after 48-96 hours of culture [26]. Based on the progress of platelet research that has been reported, it can be concluded that VEGF-A is a key factor in the regeneration process of pulp tissue which plays an important role in endodontic regenerative therapy success outcome [10, 27]. Therefore, the purpose of this study was to determine the effect of various concentrations of Advanced Platelet Rich Fibrin (A-PRF) conditioned media (CM) on the increased expression of vascular endothelial growth factor-A (VEGF-A) of hDPSCs.
2. MATERIALS AND METHODS
This study was approved by the Ethical Committee of the Faculty of Dentistry, University of Indonesia (No.82/ethical approval/FKGUI/IX/2019; Protocol Number: 070940819). Informed consent was obtained from all participating adult subjects prior to the study.
2.1. Cell Preparation and Culture
The hDPSCs used in this study were collected from ten third molars extracted from nine healthy donors who fulfilled the inclusion criteria. The donors were 18–35 years old and had no smoking and alcohol consumption habits. Immediately after extraction, molars were stored in phosphate-buffered saline (Sigma Aldrich LOT.806552, Merck, Germany). The extracted molars were split open, and pulp tissues were removed and minced into 1–2 mm pieces by using a surgical blade #20 (B Braun, Aesculap®, Germany).
The pulp tissues were then placed in 6-well cell culture plates containing Dulbecco’s modified Eagle’s medium (DMEM, Thermo Fisher Scientific Inc., Massachusetts, USA) supplemented with 10% FBS (Hyclone LOT.SH30071.02HI, Global Life Science, USA), 100 U/mL penicillin-G (Roche, Basel, Switzerland), and 100 mg/mL streptomycin (Roche, Basel, Switzerland) in a humidified atmosphere of 5% CO2 at 37 °C. At 80% confluency, cells were seeded for single-cell clone isolation. The hDPSCs were harvested at the end of the third passage (P3) and subjected to 24 h of starvation conditions in DMEM supplemented with 1% FBS. The hDPSCs (5×103 cells per well) were then seeded in four different treatments:
- Control group: hDPSCs + DMEM;
- 1% A-PRF CM group: hDPSCs + 1% A-PRF CM;
- 5% A-PRF CM group: hDPSCs + 5% A-PRF CM;
- 10% A-PRF CM group: hDPSCs + 10% A-PRF CM.
Each group had biological triplicates (Triplo) (n=3) and were observed for 5, 12, and 24 hours (three experimental observations).
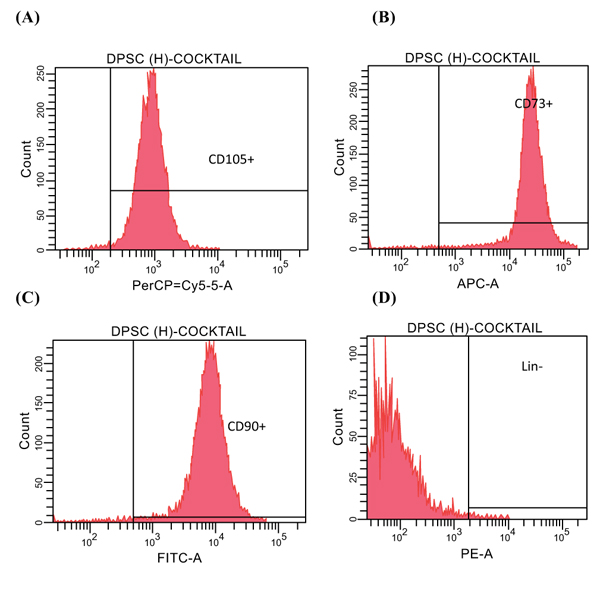
The hDPSCs were subjected to flowcytometry MSCs analysis by using FACSverse (BD Biosciences) with an MSC positive cocktail (CD 90+, CD 105+, and CD 73+) and a negative cocktail (LinNeg CD34-, CD45-).
2.2. A-PRF Conditioned Media (CM) Preparation
For blood collection, the donors were 19-35 years old who fulfilled the following inclusion criteria: no aspirin intake, no smoking and alcohol drinking habits, and not undergoing treatment with any anticoagulant. Approximately 10 ml of cubitus vein blood was collected from three donors by a certified laboratory assistant (Prodia Laboratory, Jakarta). In less than 2 min after collection, the blood samples were centrifuged at 1500 rpm for 14 minutes to obtain an A-PRF gel layer separated from red blood cells. Then, A-PRF CM was incubated at 4°C for 24 hours and diluted into 1%, 5% and 10% of A-PRF.
3. RESULTS
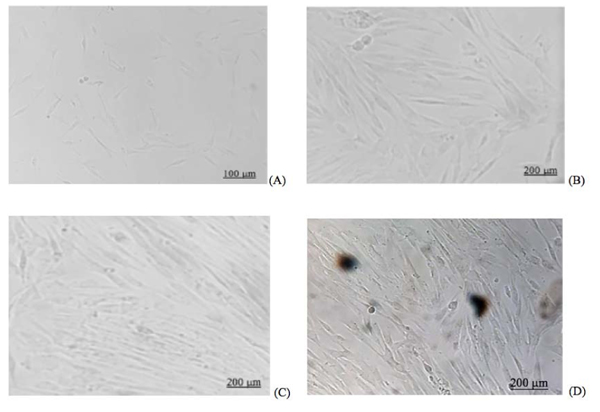
The flow cytometry results of hDPSCs are shown in Fig. (1). The microscopic morphology of the hDPSCs (Inverted microscope, Zeiss® Observer Z1, UK) is shown in Fig. (2).
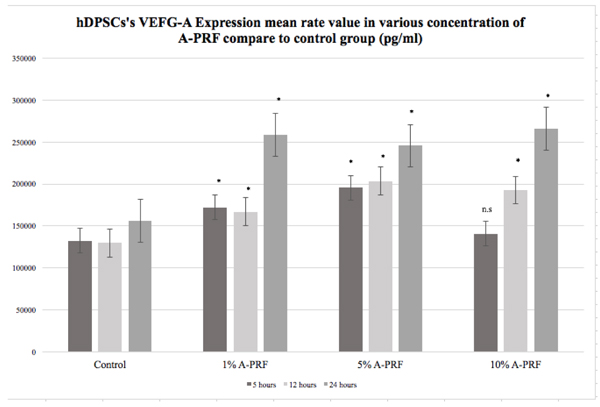
Statistical analysis using the Kruskal Wallis test showed significantly differences in VEGF-A expression of hDPSCs among all groups after 5, 12 and 24 hours of culture (p<0.05). The highest VEGF-A expression of hDPSCs at 5 hours of observation was in 5% A-PRF group (195.667 pg/ml); at 12 hours of observation, it was in 5% A-PRF group (203.667 pg/ml); and at 24 hours of observation, it was in 10% A-PRF group (266.333 pg/ml) ( (Fig. 3).
Post hoc analysis test of various concentrations of A-PRF groups (1%, 5%, and 10%) compared to control is presented in Fig. (3) (*p<0.05, post hoc Mann Whitney’s test ).
Post hoc analysis test between the various concentrations of A-PRF groups (1%, 5%, and 10%) is present in Tables 1, 2 and 3 (*p<0.05, post hoc Mann Whitney’s test). There were statistically significant differences observed between 1%, 5% and 10% A-PRF groups at 5 and 12 hours of observation (Tables 1 and 2), with 5% A-PRF showing superior VEGF-A expression of hDPSCs compared to other concentrations (Fig. 3). However, there was no statistically significant difference found between 1%, 5% and 10% A-PRF groups at 24 hours of observation (p>0.05) (Table 3), and the VEGF-A expression of hDPSCs was similar between the A-PRF groups (Fig. 3).
4. DISCUSSION
CD105+ is a glycoprotein expressed on the cell surface of human endothelial cells and is thought to play an important role in angiogenesis [5]. The hDPSCs used in the current study were subjected to flow cytometry by FACS verse (BD FACVerseTM, Biosciences USA) with an MSC positive cocktail of CD105+(97.7%), CD 90+(97.9%), and CD73+(98.6%) and a negative cocktail of LinNeg (0.5%) (Fig. 3). This result was found to be consistent with that of a previous study reporting the ability of hDPSCs in expressing some endothelial cell markers, including CD31, CD105, CD34, and von Willebrand factor, in-vitro [6]. Moreover, the result was also consistent with a statement from ISCT indicating that 95% of human MSCs can express at least three types of specific surface antigens (CD105, CD90, and CD73); therefore, the hDPSCs used in this study can be concluded to be MSCs (Fig. 1). This type of MSCs have multilineages differentiation ability, that is, they can differentiate into a number of different cells types, such as odontoblasts, osteoblasts, and endothelial cells. Therefore, the hDPSCs used in this study should be assessed in accordance with ISCT findings (Fig. 1) [1-4].
| Conditioned Media (CM) | p-value |
|---|---|
| 1% A-PRF vs. 5% A-PRF 1% A-PRF vs. 10% A-PRF 5% A-PRF vs. 10% A-PRF |
0.000* 0.000* 0.000* |
| Conditioned Media (CM) | p-value |
|---|---|
| 1% A-PRF vs. 5% A-PRF 1% A-PRF vs. 10% A-PRF 5% A-PRF vs. 10% A-PRF |
0.000* 0.000* 0.000* |
| Conditioned Media (CM) | p-value |
|---|---|
| 1% A-PRF vs. 5% A-PRF 1% A-PRF vs. 10% A-PRF 5% A-PRF vs. 10% A-PRF |
4.000 4.000 4.000 |
Platelet-derived conditioned media (CM) and their modifications can be used as alternatives to culture media with 10% FBS for most animal and human cell cultures [11-13]. Fetal Bovine Serum (FBS) may elicit severe immunological reactions against xenogeneic serum antigens, has high endotoxin content, and is a potential source of microbial contaminants, such as fungi, bacteria, viruses, and prions [12]. Consistent with the results of other studies, the hDPSCs in the current study revealed a fibroblast-like pattern in the 1% and 5% A-PRF groups seeded in four different CM after 24 hours similar to a previous study that showed fibroblast-like pattern of hDPSCs cultured in 10% FBS (Fig. 2) [11, 12]. These findings are also in line with the result from previous studies that demonstrated the potential of Platelet-derived CM as a supplement media for hDPSCs [22-24].
The hDPSCs can naturally express angiogenic protein molecules, such as VEGF, FGF-2, and MCP-1, depending on the culture media used [7-9]. In the current study, hDPSCs seeded in DMEM with no supplemented serum showed an increase of VEGF-A expression. This study showed that at 5, 12 and 24 hours, VEGF-A expression mean rate value of hDPSCs of all CM groups was increased (Fig. 3). However, no statistically significant difference was observed between 10% A-PRF group and control (DMEM) group at 5 hours of observation. These results are consistent with Tran-Hung et al. [13] who observed that 5 hours after injury, hDPSCs started to express their natural VEGF-A protein expression, which then increased at 12 hours and reached a peak at 24 hours [10].
Studies on the use of second-generation PRP and PRF as alternatives for xenogeneic-free CM for hDPSCs regeneration have evolved in the past three years [14-20]. Modifications of PRF are more practical to use because of their simple preparation procedures [14-20]. Moreover, PRF and its modifications can release some GFs, such as VEGF, FGF-2, PDGF, and cytokines [14-16]. In the current study, post-hoc Mann Whitney’s analysis test showed a statistically significant difference between 1%, 5% and 10% A-PRF groups at 5 and 12 hours of observation (Tables 1 and 2). A greater increase of hDPSCs’s VEGF-A expression was observed at 5% A-PRF group from 5 up to 12 hours of observation compared to other groups (Fig. 3). This finding showed the potential of A-PRF as an alternative conditioned media for hDPSC; thus, VEGF-A is one of the important growth factors in the angiogenesis process that underlies the continuity of dental pulp regeneration.
The collaboration of hDPSCs and endothelial cells in arteriogenesis and vasculogenesis is important in dental pulp complex regeneration. Besides that, hDPSCs can express VEGF and may induce VEGF signaling that can promote and recruit cells in differentiation and other activities related to osseointegration [21]. Bagio et al. [24] demonstrated that 1%–5% A-PRF CM could induce the DSPP expression of hDPSCs as an important growth factor in osteogenic differentiation of odontoblast after 7 days of culture [20]. In the current study, hDPSCs treated with 5% A-PRF CM could induce VEGF-A expression of hDPSCs after 5 to 12 hours, and comparable VEGF-A expression of hDPSCs was observed between 1%, 5% and 10% A-PRF groups at 24 hours of observation (Fig. 3). It can be evidenced that 5% A-PRF can induce VEGF-A and also DSPP expression of hDPSCs required in the regeneration of pulp dentinal complex because of their role in dental pulp angiogenesis and odontoblast differentiation. The A-PRF CM used in this study was prepared without any compression procedure in order to maintain the natural fibrin form. Other studies have reported significantly higher levels of VEGF and Interleukin-8 (IL-8) expression in Leukocytes-PRF (L-PRF) CM compared with L-PRF exudate (PRF with compression technique). The compression procedure in PRF-exudate preparation might interrupt the fibrin architecture, which disrupts the release of GF protein molecules over a period of time [23, 28]. This is also consistent with another study which revealed that A-PRF in fibrin mesh form can release higher Transforming Growth Factor-1 (TGF-β-1), Platelet Derived Growth Factor (PDGF) and VEGF compared to L-PRF and a significant amount of monocytes that facilitate wound healing and tissue regeneration [28]. Hence, this natural fibrin form of PRF may be capable of creating a natural environment for cells and can be an ideal autologous scaffold matrix for cells [29].
Despite the limitation of this study, we still can conclude that 5% A-PRF CM can increase VEGF-A expression of hDPSCs compared with the control group (DMEM), 1% and 10% A-PRF groups at 5 and 12 hours of observation. Although VEGF-A expression of hDPSCs at 24 hours of observation between 1%, 5% and 10% A-PRF groups was similar (Fig. 3), 5% A-PRF still showed an increase after 24 hours.
CONCLUSION
It can be concluded that 5% A-PRF CM was superior in increasing VEGF-A expression of hDPSCs at 5, 12, and 24 hours of observation. Further evaluation regarding A-PRF CM performance in the dental pulp angiogenesis process still needs to be conducted to substantiate the results of this study.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Ethical Committee of the Faculty of Dentistry, University of Indonesia (No.82/ethical approval/FKGUI/IX/2019; Protocol Number: 070940819).
HUMAN AND ANIMAL RIGHTS
No Animals were used in this research. All human research procedures were followed in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Informed consent was obtained from all participating adult subjects prior to the study.
AVAILABILITY OF DATA AND MATERIALS
The data sets used during the current study can be provided from the corresponding author (E.S) ,upon reasonable request.
FUNDING
The study was financially supported by HIBAH PUTI Doktor 2020 No.: NKB-600/UN2.RST/HKP.05.00/2020, Universitas Indonesia.
CONFLICT OF INTEREST
The authors declare no conflicts of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors thank Angliana Chouw, S. Si, M. Farm from PT Prodia Stem Cell Indonesia, for the support.


