The Effect of Self-Reported Diabetes on Alveolar Bone Loss and Number of Missing Teeth
Abstract
Background:
Diabetes mellitus, a major public health problem worldwide, is a known risk factor for periodontitis.
Objective:
This study aimed to investigate the effect of diabetes on periodontal health in a Saudi population by assessing alveolar bone level, and the number of missing teeth.
Methods:
In this retrospective study, the dental records of 203 patients (30–70 years old) patients (diabetic group = 102; control = 101) who visited King Abdulaziz University, Faculty of Dentistry, were examined through panoramic radiography. Bone loss measurements were carried out using the Ramfjord teeth index, and the number of missing teeth was counted for both groups. Independent t-test was used for comparing the total average represented by two group means, while Chi-square test was utilized to establish relationships between categorical variables.
Results:
The diabetic group had a significant 1.35-fold higher mean total bone loss (3.59 ± 1.37) compared to the control (2.66 ± 1.05). This was statistically significant in both genders (p = 001) and in >45 years old age group (p <0.05). The number of missing teeth was significantly higher in diabetic patients compared to control patients, specifically when missing >10 teeth and belonging to >55 years old age group (p <0.05).
Conclusion:
Our findings have shown a positive association between periodontal disease and diabetic patients, emphasizing the importance of early screening and diagnosis of diabetes and periodontitis in Saudi Arabia, which would help patients to avoid alveolar bone and tooth loss at early stages.
1. INTRODUCTION
Periodontal diseases are infections of the tissues and bones surrounding the tooth that affect up to 50 percent of the world-wide population [1, 2]. There are two major categories of periodontal diseases. The first is gingivitis, which is associated with dental plaque formation and characterized by the presence of clinical signs of inflammation that are confined to the gingiva and associated with teeth showing no attachment loss. The second is periodontitis, which is an inflammatory disease of the supporting tissues of the teeth caused by specific groups of microorganisms, resulting in progressive destruction of the periodontal ligament and alveolar bone with pocket formation, recession, or both [3]. Periodontitis can result in loss of alveolar bone and connective tissue surrounding the teeth and ultimately tooth loss [2, 4].
Diabetes is a group of metabolic diseases characterized by hyperglycemia resulting from defects in insulin secretion, insulin action, or both. The chronic hyperglycemia of diabetes is associated with long-term damage, dysfunction, and failure of different organs, especially the eyes, kidneys, nerves, heart, and blood vessels [5]. An increase in periodontal destruction is often noticed in uncontrolled diabetic patients compared to controlled diabetic or even non-diabetic patients. Thus, this is attributed to several factors that ultimately lead to the inability of the cells to destroy bacteria as well as exaggerated immune-mediated host tissue destruction [6]. Neutrophils, monocytes, and macrophages typically have an altered function in diabetic patients. Neutrophils often have an impaired adherence, chemotaxis, and phagocytosis. In addition, macrophages and monocytes often increase the production of pro-inflammatory cytokines such as tumor necrosis factor α (TNF-α), which increases host tissue destruction [7]. These inflammatory cytokines have a direct reaction to the supporting structures of the periodontium [8].
Several recent studies have investigated the prevalence of diabetes in Saudi Arabia, and all agree that the percentage is alarming and increases with age, reaching an average of up to 46 percent in ages >50 years old [9-12]. The relationship between diabetes and periodontal diseases is undebatable. Diabetic patients show an increase in severity, extent, and prevalence of gingivitis and periodontitis compared to healthy patients [6, 13, 14]. Thus, the aim of this study was to investigate the effect of self-reported diabetes on periodontal health in a Saudi population by assessing the alveolar bone level and the number of missing teeth.
2. MATERIALS AND METHODS
2.1. Study Population
Our retrospective study examined the dental records of patients who visited King Abdulaziz University, Faculty of Dentistry, between 2010 and 2013. Panoramic radiographs were randomly examined and a total of 203 patients (diabetic group = 102, control group = 101) with an age range from 21–70 years old were included in this study. Inclusion criteria included no systemic disease for the healthy category patients and no systemic disease except diabetes for the diabetic category patients. In addition, patients must be dentulous or partially edentulous and have no history of smoking.
2.2. Data Collection
This research was approved by the research ethical committee review board from King Abdulaziz University, Faculty of Dentistry (Ref. #009-13). This project was approved by the committee and was in full accordance with the World Medical Association Declaration of Helsinki.
The number of missing teeth was counted on panoramic radiographs in order to find the relationship between diabetes and the number of missing teeth, and then measurements of bone loss were carried out on six teeth (#16, #21, #24, #36, #41, and #44) known as the Ramfjord teeth [15, 16]. Some teeth were excluded due to lack of visibility of anatomical landmarks, cemento-enamel junction, or alveolar bone level. In addition, teeth with compromised cemento-enamel junction due to the presence of restorations, prostheses, or overlapping teeth were also excluded. Bone loss measurements were carried out by one examiner using Kodak Dental Imaging Software, Version 6.12.11.0, as an analyzing tool. Two measurements were recorded for each tooth: The amount of alveolar bone loss measured from the cemento-enamel junction to the bone crest on the mesial aspect of the mesial root and also on the distal aspect of the distal root. The measurements were summed up for each tooth and the average was calculated for each patient.
2.3. Statistical Analysis
This study was analyzed using IBM SPSS version 23 (IBM Corp., Armonk, NY, USA). Simple descriptive statistics were used to define the characteristics of the study variables through a form of counts and percentages for the categorical and nominal variables, while continuous variables were presented by mean and standard deviations. A normality test was conducted on missing teeth and showed that the distribution is not normal. To correlate the missing teeth to the demographical data, a chi-square test was used. The overall average of mesial, distal, and average of mesial and distal was determined by the average of all teeth. Independent t-test was used for comparing the total average represented by two group means. These tests were done with the assumption of normal distribution. Welch’s t-test was used as an alternative test. Last, a conventional p-value <0.05 was the criteria to reject the null hypothesis.
3. RESULTS
In this retrospective study, the effect of diabetes as compared to a healthy control group on periodontal health of 203 patients was evaluated through alveolar bone loss and the number of missing teeth assessment of their panoramic radiograph records. The number of participants was almost equally distributed in diabetic (56.2%, n = 102) and control (49.8%, n = 101) groups, whereas 52.7 % were males and 47.3 % were females (Table 1). The participants’ average age was 47.65 ± 11.1 (min = 21, max = 70). All details of demographic data are presented in Table 1.
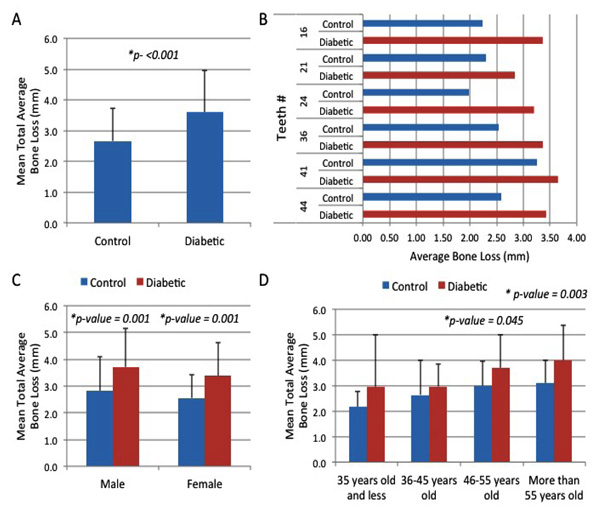
Our results reveal that the diabetic group had a significant 1.35-fold higher mean total bone loss (3.59 ± 1.37) compared to the control group (2.66 ± 1.05) as determined by the six Ramfjord index teeth (#16, #21, #24, #36, #41, and #44) (p<0.001) (Fig. 1A). The distribution of average alveolar bone loss per Ramfjord tooth is shown in Fig. (1B). The average alveolar bone loss for both groups was highest at tooth #41 (Mean bone Loss = 3.43) for the diabetic group, Mean bone Loss = 2.59) for the control group, while it was lowest at tooth #21 (Mean bone Loss = 3.36) for the diabetic group and at tooth #24 (Mean bone Loss = 2.24) for the control group (Fig. 1B). To assess whether the effect of diabetes on alveolar bone loss is reflected in both genders, the results were separated accordingly (Fig. 1C). The male diabetic group had a significant 1.32-fold higher mean alveolar bone loss (3.71) compared to the male control group (2.82) (p =001) (Fig. 1C). Similarly, the female diabetic group showed a significant 1.33-fold higher mean alveolar bone loss (3.38) compared to the female control group (2.55) (p =001) (Fig. 1C). This suggests that gender is not a factor in the assessment of significant bone losses for diabetic (p =0.245) and control (p =0.216) groups (Fig. 1C). Additionally, the association of total average bone loss relative to the age bracket for both groups was determined. When comparing different age groups, the results showed a generalized overall increase in the mean alveolar bone loss in the diabetic group compared to the control group as age progresses; however, our results revealed significantly higher total mean alveolar bone loss (3.69 and 4.00) in diabetic patients compared to controls only for the age groups of 46-55 years old (mean alveolar bone loss = 3.00) (p = 0.045) and >55 years old (mean alveolar bone loss = 3.10) (p = 0.003) (Fig. 1D). When analyzing the number of missing teeth of all participating patients, the data revealed statistical skewness to the right (1.702) and kurtosis value of 2.439 (data not shown). This suggests that the distribution of patients was not normal, thereby allowing the use of non-parametric analysis such as chi-square test to appropriately evaluate the association of missing teeth toward the demographic factors. The association of missing teeth with demographic characteristics is shown in Table 2. Results revealed significant differences in the number of missing teeth for both groups with respect to the case type (p = 0.001), suggesting that the number of missing teeth was significantly higher in diabetic patients than in the control group. Specifically, there was a significant number of missing teeth in diabetic patients in the more than 10 missing teeth group (24.5%, n = 25) compared to the control group (5.0%, n = 5) (Table 2). In addition, significant differences were observed in the number of missing teeth in both groups relative to the age group of more than 55 years old (p = 0.040), according to a chi-square test at 0.05 level. However, no significant differences were found in the number of missing teeth for both groups with respect to the age group of 55 years old and below, suggesting that age bracket is not a significant factor in the assessment of its association with missing teeth if the age is below 55 years old. Lastly, results also showed that the number of missing teeth was found to differ significantly between the two groups with respect to the male gender (p = 0.040; Table 2). However, no significant differences in the number of missing teeth were found for females (p> 0.05) (Table 2).
| Demographics | Count | % | |
|---|---|---|---|
| Total | 203 | 100 | |
| Case | Control | 101 | 49.8 |
| Diabetic | 102 | 50.2 | |
| Age | 35 years old and less | 39 | 19.2 |
| 36-45 years old | 52 | 25.6 | |
| 46-55 years old | 52 | 25.6 | |
| More than 55 years old | 60 | 29.6 | |
| Mean ± SD / Min-Max | 47.65 ± 11.1 | 21-70 | |
| Gender | Male | 107 | 52.7 |
| Female | 96 | 47.3 | |
| Variables | Total | Missing Teeth | p-value | |||||
|---|---|---|---|---|---|---|---|---|
| No Missing Teeth | 1-3 Teeth | 4-5 Teeth | 6-10 Teeth | More Than 10 Teeth | ||||
| Case | Control | 101 | 26(25.7%) | 46(45.5%) | 10(9.9%) | 14(13.9%) | 5(5.0%) | 0.001a |
| Diabetic | 102 | 15(14.7%) | 35(34.3%) | 12(11.8%) | 15(14.7%) | 25(24.5%) | ||
| Age | ||||||||
| 35 years old and less | Control | 30 | 13(43.3%) | 15(50.0%) | 1(3.3%) | 1(3.3%) | 0(0.0%) | 0.089 |
| Diabetic | 9 | 4(44.4%) | 2(22.2%) | 2(22.2%) | 0(0.0%) | 1(11.1%) | ||
| 36-45 years old | Control | 31 | 5(16.1%) | 14(45.2%) | 5(16.1%) | 5(16.1%) | 2(6.5%) | 0.741 |
| Diabetic | 21 | 3(14.3%) | 12(57.1%) | 1(4.8%) | 3(14.3%) | 2(9.5%) | ||
| 46-55 years old | Control | 20 | 3(15.0%) | 11(55.0%) | 3(15.0%) | 2(10.0%) | 1(5.0%) | 0.473 |
| Diabetic | 32 | 5(15.6%) | 12(37.5%) | 3(9.4%) | 6(18.8%) | 6(18.8%) | ||
| More than 55 years old | Control | 20 | 5(25.0%) | 6(30.0%) | 1(5.0%) | 6(30.0%) | 2(10.0%) | 0.040a |
| Diabetic | 40 | 3(7.5%) | 9(22.5%) | 6(15.0%) | 6(15.0%) | 16(40.0%) | ||
| Gender | ||||||||
| Male | Control | 41 | 11(26.8%) | 16(39.0%) | 5(12.2%) | 8(19.5%) | 1(2.4%) | 0.029a |
| Diabetic | 66 | 10(15.2%) | 20(30.3%) | 9(13.6%) | 10(15.2%) | 17(25.8%) | ||
| Female | Control | 60 | 15(25.0%) | 30(50.0%) | 5(8.3%) | 6(10.0%) | 4(6.7%) | 0.177 |
| Diabetic | 36 | 5(13.9%) | 15(41.7%) | 3(8.3%) | 5(13.9%) | 8(22.2%) | ||
4. DISCUSSION
With speculation that the prevalence of diabetes in Saudi Arabia will increase in the coming years [12, 17], this study emphasizes the need for health care providers to educate society about the importance of periodically monitoring both medical and dental health through regular check-ups, even without an obvious chief complaint. A recent study conducted among a sample population in Jeddah, Saudi Arabia, revealed that adequate knowledge of participants was noted regarding diabetes-related oral health issues; however, the majority of the participants did not receive enough information on this topic from any healthcare provider [18].
Previous research has shown a high prevalence of reported medical conditions, especially hypertension and diabetes, among individuals with periodontitis in Saudi Arabia [19, 20]. However, further studies with a larger sample size on the national level are needed to generalize these findings. In addition, the ultimate effect of diabetes on dental health is still to be further elucidated.
In this study, we used the six Ramfjord index teeth to measure the amount of alveolar bone loss as indicative of periodontal health. Previous studies in Georgia [21] and Kuwait [22] have shown the reliability of using this index in the measurement of periodontal status. Our results demonstrated an overall increase in alveolar bone loss and the number of missing teeth in diabetic patients when compared to healthy patients. This is in agreement with another study carried out by Al-Emadiet al. in sample patients at the Case Western dental clinic, Cleveland, Ohio, USA, which compared the prevalence of self-reported systemic diseases among 420 patients with or without alveolar bone loss. In comparing the patients’ radiographs, they found that subjects with moderate to severe alveolar bone loss had increased prevalence of systemic diseases, especially diabetes and hypertension [23]. Similarly, Saito et al. confirmed the same observation in a sample from the Japanese population, especially in males [24]. This seems to be associated with increased levels of C-Reactive Protein (CRP), particularly in male individuals in the Japanese population [25].
The results in our study were also consistent with other studies in that as age progresses, it may be an associated factor for periodontal disease [26-28]. In addition, our study demonstrated an association between diabetes and the number of missing teeth, especially at age above 55 years and in male individuals. Similarly, others have shown that the risk of tooth loss was higher among diabetic patients compared to non-diabetic individuals [28, 29]. Moreover, increased visits to a dental office positively lowered the risk of diabetes on the number of teeth lost [29]. Importantly, a recent study conducted by Liljestrandet al. demonstrated that an increased number of missing teeth (≥5 missing teeth) was associated with an increased risk of cardiovascular diseases, diabetes, and even death from any cause (≥9 missing teeth) [30]. This is an alarming result from our perspective, as in our tested cohort, the combined percentage of missing teeth for both diabetic and controls, even for missing ≥5 teeth, is high. This emphasizes the importance of educating our society about the impact of regular dental check-ups for both dental and general health status. We recommend the development of educational champions or programs directed toward raising knowledge and awareness of the interplay between dental and systemic diseases, including diabetes and its impact on oral and general health. This should be targeted to both healthcare workers and the general population to reach an ideal understanding of the situation.
Some important limitations of our study are the small sample size and the confined sub-population of patients who visited King Abdulaziz University’s dental school. Patients who visit the dental school are usually of lower income and education level than the general population. It has been reported that socioeconomic status may be a participating factor for periodontal disease status, as individuals with lower socioeconomic status may present with more advanced periodontal diseases compared to the rest of the population [25]. Nevertheless, our study, like others from different populations, suggests a positive association between diabetes and periodontal disease, which creates grounds for further investigation and provides a motivational tool for educating our community.
CONCLUSION
Data obtained from our study have shown a positive association between periodontal disease and diabetic patients in Saudi Arabia. This emphasizes the importance of early screening and diagnosis of diabetes and periodontitis in Saudi Arabia, which will help patients to avoid both tooth and alveolar bone loss at early stages.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This research was approved by the research ethical committee review board from King Abdulaziz University, Faculty of Dentistry (Ref. #009-13), Saudi Arabia.
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Consent form was issued to individual patients for participation.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
This project was funded by the Deanship of Scientific Research (DSR) King Abdulaziz University, Jeddah, Saudi Arabia, under grant no. 255/165/1433.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
This project was funded by the Deanship of Scientific Research (DSR) King Abdulaziz University, Jeddah, Saudi Arabia, under grant no. 255/165/1433. The authors, therefore, acknowledge with thanks to DSR for their technical and financial support.


