All published articles of this journal are available on ScienceDirect.
Comparison of Benzydamine Hydrochloride Mouthwash 0.15% and Ibuprofen in Reducing Postoperative Pain during 24 hours after Crown Lengthening: a Randomized Clinical Trial
Abstract
Background:
Crown lengthening surgery is one of the most common periodontal surgeries. The analgesic effects of benzydamine and ibuprofen tablets have been proven in various studies. The purpose of this study was to compare benzydamine hydrochloride mouthwash 0.15% with ibuprofen in decreasing the pain of patients with crown lengthening surgery who had referred to the Periodontology Department of Yazd Dentistry School in 2015.
Materials & Methods:
In this clinical trial study, 36 patients aged 30 to 60 years who referred to the Periodontal Department of the Dental Faculty of Yazd University of Medical Sciences needing a crown lengthening surgery were randomly allocated to two groups. The patients of the first group were asked to wash their mouth using benzydamine hydrochloride mouthwash 0.15% after the surgery according to the instructed protocol and the patients in the second group were asked to take an ibuprofen tablet (400 mg) every 6 hours. After 24 hours, the level of pain was measured by using the numerical criteria of the Visual Analog Scale (VAS) and the data were analyzed using SPSS software version 18. T test and Mann-Whitney test were used as appropriate.
Results:
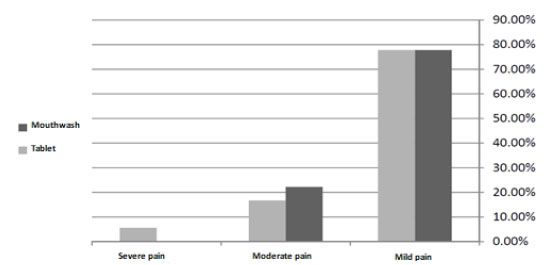
77.8% of the participants in the first group had mild pain and rest of the participants had moderate pain. 77.8% of the participants who used ibuprofen tablet reported mild pain, 16.7% had moderate pain and 5.6% had severe pain. The results did not illustrate more effect of ibuprofen on the reduction of pain after crown lengthening surgery compared with benzydamine hydrochloride 0.15% (P=0.48).
Conclusion:
In the present study, there were no changes in the VAS index between the two groups. Therefore, in order to decrease pain after periodontal surgery, benzydamine hydrochloride mouthwash can be widely used as it has fewer side effects, lower price, and similar effects with ibuprofen.
Clinical Trial Registration Code
IRCT2016012312847N2.
1. INTRODUCTION
A proper relation between restoration margin and periodontal tissues is required to maintain periodontal health. There is a need for crown lengthening aimed at biological width owing to various reasons such as dental caries, dental fractures, or problems arising during endodontic treatment [1]. It is vital to manage the patients’ pain after surgery, like any other dental operations [2]. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) are the most commonly used pain killers after a dental operation [3]. Ibuprofen is one of the most widely used pain killers in this group [4]. NSAIDs have many gastrointestinal effects due to the inhibition of Prostaglandins (PGs) production. PGs have a protective role in the digestive system, and lack of their production leads to abnormalities or even gastrointestinal bleeding. Although the enteric coat forms can decrease the possibility of indigestion, they do not hinder digestive tissue destruction and gastrointestinal bleeding [5].
In order to control pain after surgery, it is also possible to use painkilling mouthwashes, such as benzydamine hydrochloride. Benzydamine is a non-steroidal anti-inflammatory and local anesthetic mouthwash, which quickly displays its analgesic and anti-inflammatory effects. It has rare side effects, which mostly include numbness and mucosal irritation [6, 7]. In addition to the conducted studies in the field of dentistry, the analgesic effect of benzamine has also been studied in medicine. Hung and colleagues showed that spraying this substance into endotracheal cuffs led to a reduction of sore throat emergence and its severity after operation [8]. Sheibani and co-workers reported the efficacy of using the mouthwash form of this substance as well in decreasing the severity of mucositis induced by radiotherapy in patients with head and neck tumors [9]. Karavana clinically and histologically investigated the effect of gel containing benzydamine hydrochloride on repairing oral wounds in animals and showed that the gel was effective in the rapid healing of wounds [10].
Benzydamine mouthwash is an alcoholic soluble hydrochloride salt (9.6%) recognized formally by the American Dental Association (ADA) and was introduced for the first time in the 1960s, entitled 'Tantum' [7]. Some studies have assessed the relieving effects of this mouthwash in various conditions including after periodontal surgeries.
Nettis and colleagues reviewed the effects of this mouthwash as an alternative to non-steroidal anti-inflammatory medications in patients with a proven history of allergy to such drugs. They found that benzydamine hydrochloride mouthwash had an acceptable anti-inflammatory and analgesic effect, and merely resulted in acute systemic hives in 2% of the understudy population [11].
The research of Abed and others showed a reduction in the indexes of gingival bleeding. They noticed gum swelling in the group who used benzydamine mouthwash 0.15% compared with the control group [12]. Moreover, Seshan and colleagues suggested that the decline in gingival index and plaque index after using benzydamine mouthwash 0.15% was comparable with using chlorhexidine mouthwash 0.2% [13].
Surgery for crown lengthening is regarded as one of the most common periodontal surgeries and the analgesic effects of benzydamine mouthwash and ibuprofen have been proven in various studies. However, in different studies, there is no unanimous agreement on their efficacy and superiority. Therefore, we decided to compare benzydamine hydrochloride mouthwash 0.15% and ibuprofen in reducing pain after crown lengthening surgery in patients who referred to the Department of Periodontics affiliated to Yazd Dentistry School in 2014-2015.
2. METHODOLOGY
Ethical approval for this study was obtained from the Ethics Committee of the Shahid Sadoughi University of Medical Science and the study was registered in the Iranian Registry of Clinical Trials (IRCT) with the number IRCT2016012312847N2. The study was implemented on patients (age range, 20-40 years) requiring a crown lengthening surgery who were referred to the Periodontal Department of the Faculty of Dentistry affiliated to Shahid Sadoughi University of Yazd. Considering the confidence level of 95% and power of 80% as well as considering the standard deviation of 3.2 for after surgery pain, the minimum sample size was calculated in each group as 18 patients.
The inclusion criteria were as follows: 1) patients requiring crown lengthening surgery in one of the maxillary premolar teeth, 2) adequate keratinized gingiva in the site of surgery, 3) acceptable crown/root ratio after crown lengthening surgery, and 4) systemic health.
On the other hand, the exclusion criteria were: 1) moderate to severe periodontitis, 2) patients with diabetes, cardiovascular disease, and hypertension, 3) cigarette smoking, 4) prohibition of NSAIDs intake, 5) use of various anti-inflammatory drugs within the previous 6 months, 6) pregnancy, and 7) using extra pain killers during 24h after surgery due to uncontrollable pain.
Informed consent was obtained from the participants. When the first stage of periodontal treatment was completed and necessary examinations were performed, surgical time was determined for the patients. All the surgeries were conducted by a surgeon in the specialized department of Yazd Dentistry School. The surgical technique used for all the patients was alike. The flaps were apically displaced accompanied by osteotomy. In the end, the flap was sutured by using simple loop sutures on the two sides of the teeth with a 3-0 silk thread. After asking about the history of allergy to penicillin, amoxicillin (500-mg capsules) was prescribed. After the completion of the surgery, periodontal dressing was not used in the area so that the benzydamine mouthwash may be effective.
The concept of pain severity and the method of measuring it after the surgery were explained by a visual analog scale (VAS) method for the patients at the time of referring. The consent form was filled in by the patients and they were asked to indicate the rate of pain within the first 24 hours after the surgery in the operated area according to the specifications of the questionnaire as a number from 0 to 10 showing mild pain (0-4), moderate pain (4-7), and severe pain (equal and above 7).
The patients were equally and randomly divided into two groups by random digits table. They were instructed about the correct method of using mouthwash and tablets. The first group was asked to wash their oral cavity using 15 ml of benzydamine hydrochloride mouthwash 0.15% (Exir Boroujerd Pharmaceutical Company) every 3 hours for 30 seconds. Proper use of mouthwash was checked by the researcher before surgery. Also, the patients were told to take painkillers if there was uncontrollable pain. The second group was asked to take one tablet of ibuprofen 400 mg every 6 hours (Hakim Pharmacy Company). Educating and evaluating the patients on how to use mouthwash properly and ingest tablets were the basic limitation of the plan. Using of tablet and the mouthwash was reminded by researcher in the first 8 hours after surgery.
Data were analyzed by a blinded third person using SPSS software version 22 using t test and in case of abnormality of the pain scores, Mann-Whitney test was used (α=0.05).
3. RESULTS:
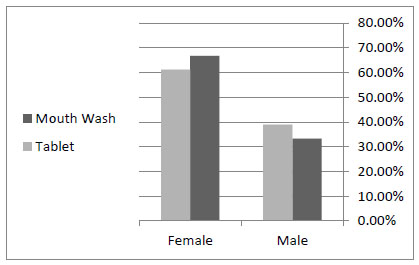
Seven patients out of the 18 in the control group were male and 11 of them were female, with an average age of 34.6 ± 11.2. Among the 18 patients in the case group, there were 6 men and 12 women, with an average age of 32 ± 7.8. According to t test, mean of age and sex distribution between the control and case groups had no statistically significant difference (Table 1, Fig. (1)).
| SD | Mean | number | Groups |
|---|---|---|---|
| 11.2 | 34.6 | 18 | Control group |
| 7.8 | 32 | 18 | Case group |
According to the Chi square test, there was no significant difference between the score of VAS index in the mouthwash and tablet groups (P=0.48, Fig. (2)).
According to Table 2, and based on the Mann-Whitney test and the mean score, there was no statistically significant difference in pain between the control and case groups (P=0.34).
4. DISCUSSION
This study compared the analgesic effects of benzydamine mouthwash and ibuprofen tablets. Despite reporting severe and moderate pain by some patients, none of them used additional painkillers during 24 hours after surgery and adhered to the research protocol.
| SD | Mean | number | Groups |
|---|---|---|---|
| 2.31 | 2.77 | 18 | Control group |
| 1.45 | 3 | 18 | Case group |
Benzydamine hydrochloride mouthwash have inhibitory effects on various stages of producing arachidonic acid cascade. It rapidly indicates its anti-inflammatory and analgesic effects [14].
Studies conducted on the effect of benzydamine on human gingival fibroblasts led to the conclusion that benzydamine results in reducing the production of inflammatory cytokines such as PGE2 and 6-keto-PGF1α, as well as decreasing the effect of IL-1β and TNFα, along with inhibiting the production of arachidonic acid through impeding cyclooxygenase and phospholipase A2 [15]. Ibuprofen tablets were chosen for the control group due to the highly prevalent use of this drug after surgical treatment and its definite analgesic effects in accordance with the previous different studies [16].
In the present study, there was no significant change in VAS indices between the two groups. Only one of the patients who was a 32-year-old lady reported nausea and dizziness after using mouthwash. Therefore, in order to reduce pain after periodontal surgeries, benzydamine hydrochloride 0.15%, which has fewer side effects as well as low cost, and similar effectiveness as ibuprofen 400 mg can be used. In addition, studies showed that mouthwash was effective in decreasing inflammation, and particularly in reducing the gingival bleeding index. Furthermore, Herrera similarly showed that using this mouthwash in combination with cetylpyridinium chloride can be effective temporarily to stop plaque formation [17].
Due to the adverse effects of NSAIDs, many studies try to find a substitute for this group of drugs.
Diclofenac mouthwash is another analgesic mouthwash that has been studied. Roobal Behal showed that the new 0.074% diclofenac mouthwash was an effective and tolerable medicinal product for post-surgical symptomatic relief in periodontal surgeries [18].
Nasrinzadeh and colleagues also studied the effect of using benzydamine mouthwash following periodontal flap surgery. The authors stated that using this mouthwash could reduce pain after surgery, gingival bleeding, and plaque accumulation compared with placebo [19].
In the study of Khoshkoonejad and others, the relieving effect of benzydamine mouthwash and acetaminophen codeine was compared in patients requiring a periodontal surgery. This study showed that benzydamine was not as effective as acetaminophen codeine in reducing pain after surgery in case the patients are not divided based on their disease severity or the amount of conducted bone correction. But after classifying the patients in chronic primary periodontitis group, moderate chronic periodontitis, osteoplasty, ostectomy, and bone correction groups, it was found that benzydamine had effects in mild chronic periodontitis, osteoplasty, and lack of bone correction comparable with acetaminophen codeine [20]. Regarding the patients with chronic moderate periodontitis or in need of ostectomy, it can be said that due to the extent of the implemented surgery, lack of effective pain-killing with benzydamine mouthwash is justifiable.
The study of Khorshidi and colleagues also illustrated that using benzydamine hydrochloride mouthwash in comparison with 500 mg acetaminophen after surgery on soft tissue led to an equal analgesic effect [21].
Goswami and colleagues reviewed the effect of using benzydamine hydrochloride mouthwash after removing the mandibular third molar among 40 patients. They reported that despite a reduction in the need for oral painkiller following the use of this mouthwash compared with the control group, this decline was not statistically significant [22].
The reason for the difference between this result and our findings, considering the similarity of the sample sizes, could be a result of the type of operation, which has been crown lengthening surgery in our study whereas in the Goswami study, the removal of the third molar had been performed.
Levent Sigermi studied the analgesic effects of naproxen sodium–codeine phosphate in combination, diclofenac potassium, and benzydamine hydrochloride (in coated pill form) after third molar extraction surgery. He found that benzydamine hydrochloride had similar efficacy to diclofenac potassium and both were less effective than naproxen sodium–codeine phosphate in pain reduction [23]. They concluded that naproxen sodium–codeine phosphate could be the drug of choice after the extraction of a patient's impacted lower third molar and benzydamine hydrochloride could be used as an NSAID [23].
CONCLUSION
There was no significant difference in VAS scores between the two groups using benzydamine hydrochloride 0.15% and ibuprofen 400-mg tablets. Therefore, it is recommended to use benzydamine hydrochloride 0.15% to reduce pain after periodontal surgeries, which has lower side effects and cost as well as the same efficacy.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This clinical trial was approved by Ethics Committee of Shahid Sadooghi University of Medical Sciences, Yazd, Iran (IR.SSU.REC.1394.30).
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures were followed in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
The participant provided written informed consent.
STANDARDS OF REPORTING
This report follows the protocol established by CONSORT Statement. The study was registered in the Iranian Registry of Clinical Trials (IRCT) with the number IRCT2016012312847N2.
AVAILABILITY OF DATA AND MATERIALS
The authors confirm that the data supporting the findings of this study are available within the article.
FUNDING
None.
CONFLICTS OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors sincerely wish to thank all the colleagues at the Periodontal Department of the Faculty of Dentistry at the Shahid Sadoughi University of Yazd, Iran, for their support.


