All published articles of this journal are available on ScienceDirect.
The Prevalence of Streptococcus Mutans with Different ABO Blood Groups Among Healthy College Students
Abstract
Objective:
This study was conducted to determine the correlation between the occurrence of Streptococcus mutans (S. mutans) which is considered as the main pathogen responsible for the initiation and development of dental caries with blood groups and Rhesus (Rh) factor that are considered as a genetic predictor of having dental caries among healthy students.
Methods:
Saliva and blood samples were taken from 40 healthy students whose ages were between 19-23 years old in the College of Dentistry/Al-Iraqia University in Baghdad, Iraq. Estimation of the flow rate and pH was done for all the collected saliva samples within 5 min of saliva collection. Then, 100 µL of saliva was cultured on Mitis salivarius bacitracin agar (MSBA) at 37 °C for 48 h. The colonies of S. mutans were identified by their morphology and biochemical tests. Later, 1 to 2 drops of blood were taken from each student’s thumb to determine the blood group.
Results:
This study showed there was a greater prevalence of S. mutans among students of blood group A in contrast to the blood group O. In addition, Rh+ groups were dominant than Rh- groups among the study samples.
Conclusion:
S. mutans levels were higher in the blood group A followed by AB, B, O blood groups among the college students studied.
1. INTRODUCTION
Dental caries is a complex and multifactorial disease of the teeth. The development of caries in humans is affected by many factors such as host factors (saliva compositions as flow rate, viscosity, and pH of saliva), diet and the presence of acidogenic bacteria in the dental biofilm, particularly Streptococcus mutans (S. mutans). Saliva contains organic and inorganic ions, e.g. phosphate, calcium, and bicarbonate which act as important buffering agents as well as many directly and indirectly acting antimicrobial agents such as e.g. lysozymes, α- and β- defensins, lactoferrin, lactoperoxidase, agglutinin and IgA. A small amount of fluoride could also be present in saliva that can affect the initiation and progression of oral diseases. Salivary pH and flow rate are important characteristics for assessment of oral health. Many studies [1-5] focused on the levels of mutans streptococci in saliva as a predictor of the induction and progression of dental caries. Mutans Streptococci has been involved as a primary causative agent of dental caries in humans and one of its crucial virulence properties is the ability to form dental plaque which is a biofilm on surfaces of the tooth. S. mutans is found among healthy individuals as well as in caries suggesting the contribution of genetic variation on the disease development; therefore genes marker can predict the development of dental caries [6, 7].
A blood group is a genetic and inherited character. The global classification of blood grouping is the ABO system, which is discovered by Landsteiner; however, other important blood systems are the MN and Rhesus (Rh) system. The ABO and Rh systems are determined by the nature of different proteins present on the surface of red blood cells. The antigens of the ABO system are found in plasma and other body fluids and are an integral part of the red cell membrane. ABO blood groups are the most investigated erythrocyte antigen system, and they have been used as genetic markers in studies of their associations with various diseases because of the ease in the identification of their phenotypes. Several studies [8, 9] have been carried out to investigate relationships between the ABO blood groups and the incidence of oral and dental diseases. The aim of this study was to estimate the correlation between the occurrence of S. mutans with the genetic factors: blood groups and Rh factor among healthy participants.
2. SUBJECTS AND METHODS
2.1. Saliva Collection
Saliva was collected from forty healthy students in Dentistry College/Al-Iraqia University whose ages were between 19-21 years old from November 2018 to February 2019. Ethical Committee Approval was achieved for the protocol before starting the study and written informed consent was obtained from all the participants. About 2-5 mL of saliva was collected from each student after ensuring that he/she did not drink or eat at least 1 h before collecting saliva. All collected samples of saliva were estimated for pH and flow rate which were obtained in the initial 5 min of saliva collection. pH strip (Consort, Belgium) was used to estimate the pH, and the salivary flow rate was determined as the quantity of saliva collected within a specific time [10, 11].
2.2. MSBA Preparation
Mitis salivarius bacitracin agar (MSBA) (the selective media for Streptococcus mutans) was prepared according to the manufacturer’s instructions. Forty-five g of the mitis salivarius agar (MSA) media powder (Himedia, India) was dissolved in 500 mL of distilled water. Then, 75 mg was added to the mixture. Later, the mixture was autoclaved at 121 °C for 15 min and left to cool at 45 °C. Finally, 0.5 mL of sterilized bacitracin solution (with concentration 200 U/L) (Oxoid Inc., Canada) was added to the medium and then poured in several Petri dishes and allowed to cool [12, 13].
2.3. Culturing of S. Mutans
Serial dilution of concentrations (10-1- 10-5) was prepared from salivary samples. 50 µL was taken from each dilution, which was cultured on MSBA and incubated at 37°C anaerobically using the anaerobic jar for 24 h and then for 24 h aerobically. Later, the colonies of S. mutans were identified by a colony, cell morphology and some biochemical tests [14].
2.4. Morphological Characteristics
The morphology of the isolated S. mutans colonies was examined directly on the MSBA plates and by the aid of the dissecting microscope (X 15 magnification) (Olympus, Japan). S. mutans colonies determination depends on their morphological characterization on the selective agar as described by Edwardson [15]. Then the number of colonies was documented considering the dilution factor and expressed as colony-forming unit per ml of saliva (CPU/mL) [13].
2.5. Gram Stain Cell Morphology
A colony was picked up from MSBA plates under the sterilized conditions and subjected to Gram stain, according to Koneman et al. [16] and examined by the aid of a dissecting microscope.
2.6. Catalase Test
An inoculum from a pure culture of S. mutans grown on MSBA was transferred using a sterile loop to a surface of a clean dry glass slide, then one to two drops of 3% v/v hydrogen peroxide (H2O2) (PeroxyChem, USA) was added immediately to the portion of a broth on the slide. The appearance of gas bubbles is an indication of a positive catalase test [16, 17].
2.7. Identification of Blood Groups
ABO blood group phenotypes (A, B, AB, and O) and Rh factor were determined by ABO typing reagents (Diagast, France) and by employing the conventional slide agglutination method, which is based on agglutination reactions between blood group antigens and blood group-specific anti-sera on the cell surface of erythrocytes [18, 19].
2.8. Statistical Analysis
Data were analyzed using the statistical software, namely, Statistical Package for the Social Sciences (SPSS, Version 20, IBM Analytics). Microsoft Word and Excel have been used to generate graphs and tables. The t-test and One way-ANOVA tests have also been used to reveal the effect of different factors on the study parameters.
3. RESULTS
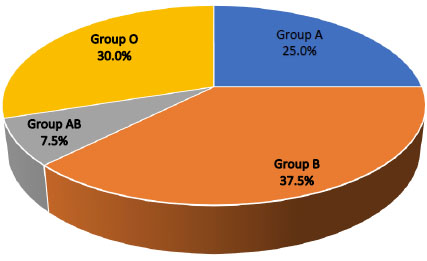
Forty healthy students of Dentistry College from Al-Iraqia University were included in this study, 60% of them were male and 40% were female, 85% were residents of Baghdad and 15% were non-Baghdad residents. No significant relationship was found between the sex and blood groups while a highly significant relationship was found between the residence and different blood groups (Table 1). According to ABO blood groups, 25%, 37.5%, 7.5% and 30% of the study groups were A, B, AB, and O, respectively. Moreover, 97.5% of the study samples were Rh+ and 2.5% were Rh- (Table 2) (Fig. 1).
3.1. Morphological Identification and Microscopic Examination of S. Mutans
On the selective MSBA plates, S. mutans colonies appeared as light blue, spherical or ovoid with raised or convex surface. Microscopical examination of S. mutans colonies with Gram stain appeared as gram-positive short or long chains with rough or frosted - glass appearance (Fig. 2).
| Variables | n | Frequency | Chi-Square | P |
|---|---|---|---|---|
| Sex Male Female Total |
24 16 40 |
60% 40% 100% |
1.22 | 0.2684 |
| Occupation Baghdad Resident Non-Baghdad Resident Total |
34 6 40 |
85% 15% 100% |
18.22 | 0.0001** |
| Blood Group | n | Frequency | P |
|---|---|---|---|
| Group A | 10 | 25% | 0.0075** |
| Group B | 15 | 37.5% | |
| Group AB | 3 | 7.5% | |
| Group O | 12 | 30% | |
| Total | 40 | 100% | |
| Rh Factor | |||
| Rh+ | 39 | 97.5% | 0.0001** |
| Rh- | 1 | 2.5% | |
| Total | 40 | 100% |
3.2. Salivary Flow Rate Among Different Blood Groups
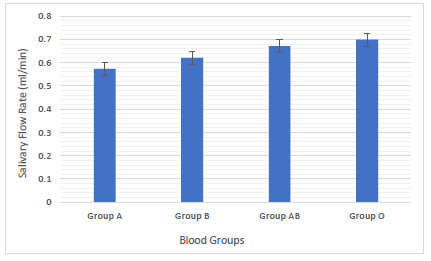
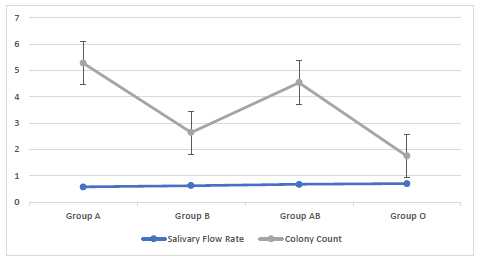
Blood group O had a higher mean salivary rate of (0.69 mL/min) followed by blood group AB (0.67 mL /min), blood group B (0.62 mL /min) and blood group A (0.57 mL /min). No significant relationship of the salivary flow rate with blood groups was found among the studied groups (Fig. 3).
3.3. Salivary pH Among Different Blood Groups
Blood group A had a higher mean salivary pH (7.2) followed by blood group AB pH (7) and blood group B pH(7) then blood group O pH (6.9). No significant relationship of the salivary pH with blood groups was found among the studied groups (Fig. 4).
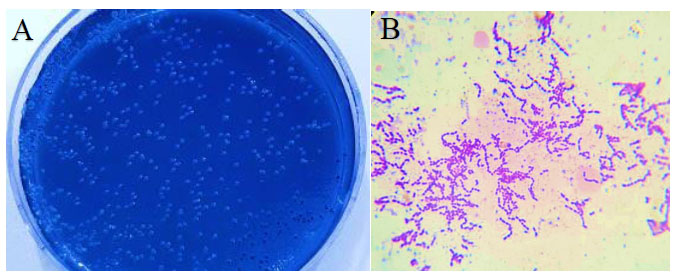
(A) Culture of S. mutans on MSBA.
(B) Gram stain of S. mutans under dissecting microscope (X 1000 magnification).
3.4. The Viable Count of S. Mutans Among Different Blood Groups
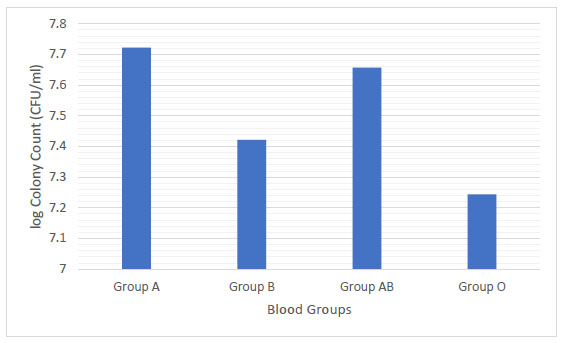
Group A had a higher mean colony count (5.27 X107 CFU/mL) followed by blood group AB (4.54 X107 CFU/mL), blood group B (2.64 X107 CFU/mL) and blood group O (1.75 X107 CFU/mL). No significant relationship of the viable count of S. mutans with blood groups was found among the studied groups (Fig. 5).
3.5. The Relationship Between pH and Viable Count of S. Mutans
A highly significant relationship was found between the two variables: pH and viable count of S. mutans among the studied blood groups (Fig. 6).
3.6. The Relationship Between Salivary Flow Rate and Viable Count of S. Mutans
A significant relationship was found between the two variables : the salivary flow rate and viable count of S. mutans among the studied blood groups (Fig. 7) and the correspondence analysis of salivary flow rate, pH and log viable count of S. mutans with blood groups (Fig. 8).
4. DISCUSSION
Dental caries is a polymicrobial oral disease. Determining the risk assessment of dental caries can help prevent the occurrence or decrease the progression of this disease. Dental caries can be affected by molecular and genetic factors [20, 21].
Saliva can be considered as a mirror for determining the general oral health because it contains hormones, antibodies, proteins and other molecules. Different pathological and physiological factors may lead to variation in the salivary flow rate. Low salivary flow rate results in clinical oral discomfort such as susceptibility to altered taste sensation as well as increased susceptibility to oral candidiasis, oral infection and dental caries. The study results revealed that group O had a higher mean salivary rate (0.69 mL/min) while group A had a lower mean salivary rate (0.57 mL/min) among blood groups (Fig. 3). Based on the results of salivary flow rate taken from healthy individuals which were less than (1.0 mL/min), these individuals were considered to be at risk of developing dental caries and should be advised to reduce the frequency of sugar ingestion as well as acceptable patterns of oral hygiene [22].
ANOVA test is used, the p-value is 0.78. Data presented as highly significant** where p<0.01 and as significant * where p<0.05 to be considered.

ANOVA test is used, the p-value is 0.31. Data presented as highly significant** where p<0.01 and as significant * where p<0.05 to be considered.
ANOVA test is used, the p-value is 0.59. Data presented as highly significant** where p<0.01 and as significant * where p<0.05 to be considered.

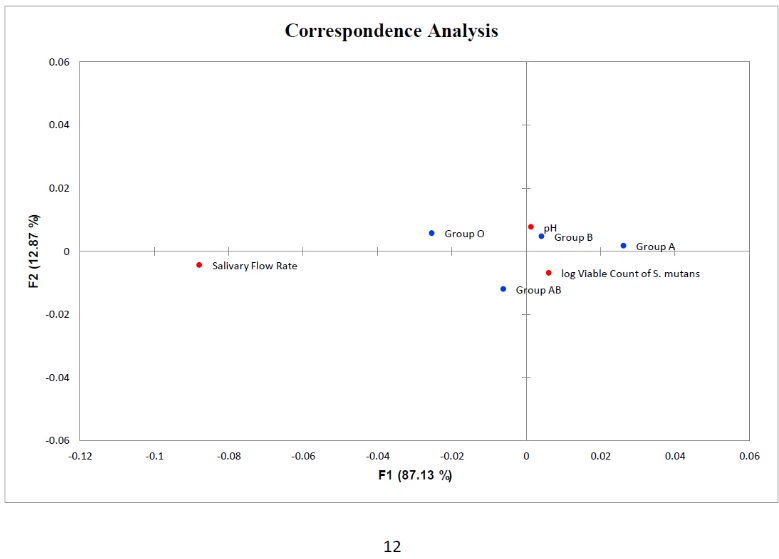
Points in red correspond to salivary flow rate, pH and log viable count of S. mutans Points in blue correspond to blood groups.
In the current study, the mean pH range among the blood groups was between (6.9-7.2) that is above the critical pH value (5-5.5) to cause demineralization and further dental caries. This is in accordance with studies of Kaur et al., and Bradshaw et al. [22, 23], that have shown individuals with low or no caries to have a salivary pH of around 7. The mean pH value was higher in group A and lower in group O (Fig. 4). The variations in these results may because all the samples were taken from healthy persons so their pH was almost neutral.
In this study, the mean colony count of S. mutans was the highest in blood group A (5.3 X 107 CFU/mL) and lowest in blood group O (1.7 X 107 CFU/mL) (Fig. 5). A significant relationship was found between the S. mutans count with salivary flow rate and pH (Fig. 6 and 7). S. mutans is one of the most common types of bacteria associated with oral diseases such as tooth decay and dental caries, so it is very critical to determine its levels [24, 25]. More than 700 species bacteria including S. mutans have been bathed in saliva. The high level of S. mutans is an indicator of caries in deciduous and permanent teeth. Moreover, the mutans streptococci has the acidogenic capacity and is able to adhere to the enamel surface, resulting in an oral cariogenic ecosystem. S. mutans is considered as transient before teeth eruption and can be easily detected. This early detection of S. mutans could be due to vertical transmission of the same bacterial genotype from mother to child [26, 27] and this is in accordance with the study by Widita et al. [28], that demonstrated a relationship between the oral hygiene of the mother with the presence of decay teeth in her children. Although no significant relationship of S. mutans levels was found with blood groups, blood groups can be considered as a potential indicator for the development of dental caries [29]. About 80-85% of individuals (called as secretors) have blood type antigens that raft freely in their body such as in saliva, semen and body fluids, while others, (non-secretors) do not secrete their blood type antigens in different liquids. Later, the emission of ABO antigens to saliva may be depressed by the capacity of microorganisms to adhere to the surface of the teeth because the microorganisms have ectins on their surface which may be attached to the body surfaces and are mostly ABO specific. On the other hand, the non-secretors individuals have the tendency to break down the immunoglobulin A (IgA) antibodies level in the saliva that may trade off their capacity to keep little bacterial count . Therefore, blood groups may process a strong predictive value and a genetic marker of the susceptibility of an individual to have oral diseases [30, 31]. Therefore; it is suspected that individuals with blood group A are more prone to have gingivitis and dental caries in contrast to blood group O individuals. This study observation is in agreement with the study by Demir et al. [32], that found a higher level of blood group type A in patients with gingivitis and a higher level of blood group O in patients with periodontitis. On the other hand, this study is in contrast with the study by Patel et al. [33] and Sahani et al. [34]. These differences in the results may be due to individuals differences and an unequal number of the blood group samples. Furthermore, the present study revealed that 97.5% of the study population were Rh+ and only 2.5% were Rh-. Similar findings have been reported by the studies of Gautam et al. [35] and Agrawal et al. [36]. A significant relationship was found between the Rh factor and ABO blood groups. Further studies are needed to confirm these results due to a small number of individuals included in this study.
CONCLUSION
The study confirms that blood group A is the commonest while blood group O was the least of the blood groups among the college students with the prevalence of Rh+ over Rh- related to the occurrence of S. mutans.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Ethics Committee of Dentistry College of Al-Iraqia University, Iraq.
HUMAN AND ANIMAL RIGHTS
No Animals were used in this research. All human research procedures were followed in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Written informed consent was obtained from all the participants.
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available from the corresponding author, [S.A], upon reasonable request.
FUNDING
None.
CONFLICT OF INTEREST
The author declares no conflict of interest, financial or otherwise.
ACKNOWLEDEGEMENTS
We are thankful to the Dentistry College/ Al-Iraqia University in Baghdad, Iraq for helping us carrying out this research to a conducive outcome.


