All published articles of this journal are available on ScienceDirect.
A New Tooth Processing Apparatus Allowing to Obtain Dentin Grafts for Bone Augmentation: The Tooth Transformer
Abstract
Introduction:
Human dentin matrix could be considered an excellent alternative to autologous or heterologous bone graft. Autologous tooth graft has been proposed since 1967 when the osteoinduction properties of autogenous demineralized dentin matrix were discovered.
Methods:
The preparation technique to transform autologous teeth in suitable grafting material still represents the fundamental step of the whole procedure.
Aim:
The aim of the present study was to test an innovative medical device that could obtain tooth graft materials starting from the whole tooth of the patient. 15 consecutive cases of tooth grafting procedures were performed with a mean follow up period of 18 months.
Results:
In all cases, after 6 months of healing, the defects were almost completely filled by newly formed hard tissue. The new tissue was examined after 6 months, both from a radiological point of view by CBCT scans and from a clinical observation. It showed a compactness similar to the medium-density bone. No signs of inflammations were observed. No infective complications were recorded during the post-operative healing. No graft particles or grains were visible in the regenerated bone structure that appeared homogeneous and uniform.
Discussion:
The results of the present study showed favorable bony healing in guided regenerative surgery procedures using autologous tooth graft. Future studies with long follow up period are needed in order to better evaluate the potential of demineralized dentin autografts.
1. INTRODUCTION
Grafting materials are widely used for pre and peri-implant bone augmentation procedures from more than 35 years [1, 2]. The most commonly used graft materials are from animal origin, synthetic or human. In these cases, bone regeneration stimulation derives from the host organism and never from the donor, slowing or decreasing the regenerative potential. Autogenous bone graft is considered the gold standard for the repair of alveolar bone defects, but it is associated with donor complications and morbidity and also suffers from a limited supply.
Permanent teeth graft has been proposed since 1967, when Yeomans and Urist [3] and Bang and Urist [4] demonstrated osteoinduction properties of autogenous demineralized dentin matrix; some years later, Kawai and Urist partially purified bone morphogenetic proteins of bovine dentin matrix [5].
The idea to use autogenous tooth as bone substitute in grafting procedure started from similar chemical composition between bone and dentin. Both of them consist, in fact, of 18% collagen, 2% proteins, 70% inorganic portion (hydroxyapatite), and 10% of fluids. Both tooth and alveolar bone are derived from neural crest cells and are made up of the same type I collagen.
In 1991, Bessho et al. [6], demonstrated the presence of Bone Morphogenetic Proteins (BMPs) from human dentin matrix. Therefore, both bone and dentin matrix is a repository for growth factors, such as Bone Morphogenetic Proteins (BMPs) and basic fibroblast growth factor. It was also reported that the demineralized human dentin matrix was able to induce bone and cartilage formation in mouse muscles with the concomitant presence of bone-forming cells (osteoblasts) [7]. The consistent number of recent studies [8, 9] existing in this topic testifies the scientific increasing interest about this possibility for grafting procedures.
The demineralized dentin matrix may represent an efficient reserve of BMPs because highly soluble BMPs do not exert osteoinductive effects when used alone. Bioactive Growth Factors (GFs), such as Transforming Growth Factor-B (TGF-B) and bone morphogenic proteins (BMPs), which are known to be present in and released from dentin, are involved in bone repairing processes [10].
The preparation technique to transform autologous teeth in suitable grafting material represents the key step of the whole procedure. It is fundamental to preserve the organic autologous components to stimulate bone progenitor cells, remove any contaminants to avoid inflammatory or infective reactions, and prepare the inorganic part to be easily colonized by osteoblasts. The demineralization process is required for freeing the various growth factors and proteins, since the release of the growth factors is sometimes blocked by the presence of hydroxyapatite crystals [11]. Through the reduction of the mineral phase, demineralization supports the release of such growth factors from the tooth matrix [12].
At present, demineralized autogenous dentin is available in two forms, granules and block [13]. Some authors [14] theorized that the geometry and granules size assume a fundamental importance in terms of bone regeneration pro-perties. Recently, Koga et al. [15], recommended the use of 1000 μm particles and a partial demineralization with 2% HNO3.
There are some devices, on the market, that allow to grind the tooth of the patient to produce dentin granules. These devices need a manual control by the clinicians in grinding process without any control in particles size. Furthermore, also the chemical treatment to obtain tooth decontamination and demineralization is entrusted to the operator's manual skills [16].
Recently, an innovative medical device (TT TOOTH TRANSFORMER SRL, Via Washington, 59 - Milan, Italy) to obtain suitable tooth graft materials starting from the whole tooth of the patient was introduced to the market. All grinding and demineralizing processes are completely automated without any human possibility of error. According to the manufacturer, this new device represents an advanced system in the area of tissue engineering that can process and transform extracted tooth into useful bone graft material in a short time. The autogenous genetic content guarantees absolute com-patibility with the recipient site and most importantly, the content of BMP-2 (Bone Morphogenic Proteins that stimulate bone growth), found by some authors in a previously published in vitro investigation [17], could guarantees high oste-oinduction.
This autologous tooth graft should be able to stimulate adhesion, proliferation and cellular differentiation and to promote the bone regeneration. The high wettability allows the ease of handling. The tooth like a bone, is made of collagen type 1 and minerals of Hydroxyapatite (HA). The tooth trans-former reduces the crystallinity of the HA, eliminates the bacteria and transforms the dentin into an autologous graft material. In vitro studies demonstrated the presence and the freeing of BMP-2 from tooth and the elevated biocompa-tibility of the dentin after treatment [18].
The present paper aims to present clinical and histological outcomes of 11 consecutive case of socket preservation and 4 cases of sinus lift using innovative autologous tooth grafting procedure.
2. MATERIALS AND METHODS
The present case series included 15 patients (7 male and 8 female), age ranged between 22 to 64 years old. All patients were in good health conditions and were nonsmokers. In 11 cases, the patients needed guided bone regeneration procedures while in other 4 cases, patients asked for maxillary sinus elevation. An informed and written consent to the surgical treatment was obtained from each subject. The approval of the ethical committee was not needed because it is a case series analysis without any experimental material or procedures (the device is present in the market). The patients were advised that the bone regeneration procedures would be made using autologous tooth as regenerative material and each patient declared to understand the benefits of using an autologous bone regeneration material.
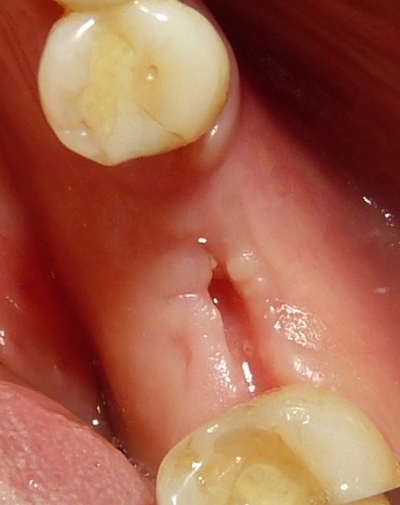
In each case, the patient underwent 3D radiological analysis (Fig. 1) before proceeding to tooth extraction and/or regeneration procedures. Three to four days before surgery, patients were prepared with an oral hygiene session and received a reinforcing oral hygiene instruction to have an appropriate cleanliness by means of electric-toothbrush. Twenty-four hours before surgery, antibiotic prophylaxis was started (amoxicillin 1g twice a day for 6 days).
In cases in which also tooth extractions were planned (9 patients out 11), patients underwent hopeless tooth extraction some minutes before starting bone regenerative procedures while in the remaining two cases deciduous or wisdom teeth, conserved by the patient over the years, were used.
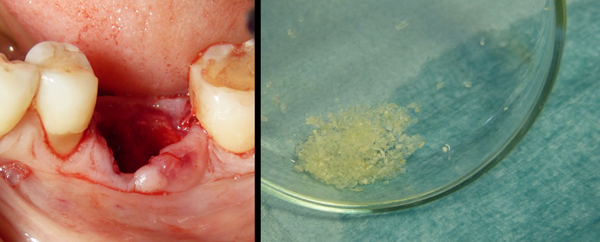
In all cases, extracted teeth were carefully cleaned and any foreign materials were removed including calculus, restorations and endodontic filling materials before its insertion in the device (Fig. 2). Cementum was not removed.
In particular, the tooth of the patient was treated for:
- Cleaning of residual calculus using piezoelectric instrument;
- Cleaning the root surface using diamond burs;
- Removing any filling materials (guttapercha, compo-site, etc.)
- Finally, it was cut in small pieces to facilitate the milling process.
Subsequently, the tooth was placed inside the mill. A small box containing disposable liquids was inserted in the device in its correct position (indicated by arrows). According to the manufacturer, these solutions guarantee maximum release of BMP-2 and collagen as well as a decontamination of the root. When all the components were inserted and the cover of the machine was closed, the device was started using the general switch button. Demineralized dentin graft was ready in 25 minutes to be placed in the patient’s mouth (TT TOOTH TRANSFORMER SRL, Via Washington, 59 - Milan, Italy).
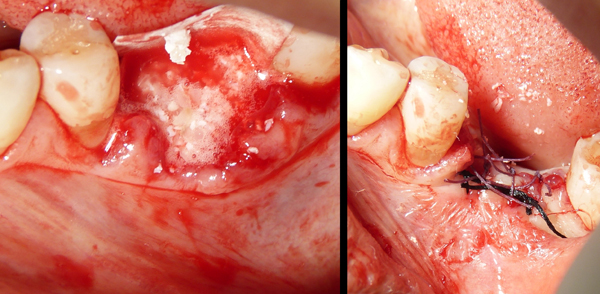
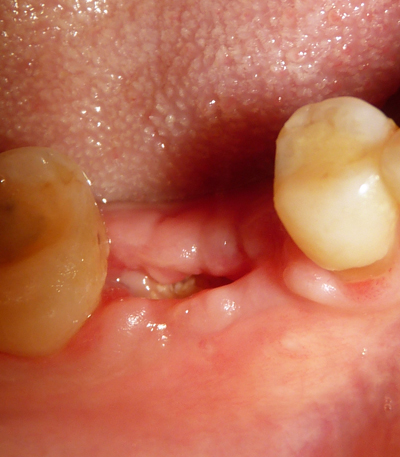
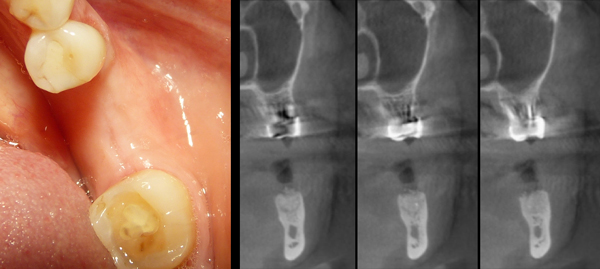
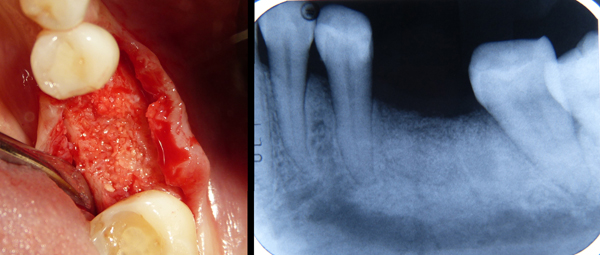
In all cases, the graft was covered by a resorbable porcine pericardium membrane (BEGO Implant Systems GmbH & Co. KG, Wilhelm-Herbst-Straße, Bremen, Germany) (Fig. 3). An immediate post-operative radiological check was performed (Fig. 4). Each patient underwent clinical examination after 10 and 30 days (Fig. 5) and (Fig. 6) in order to evaluate the healing process.
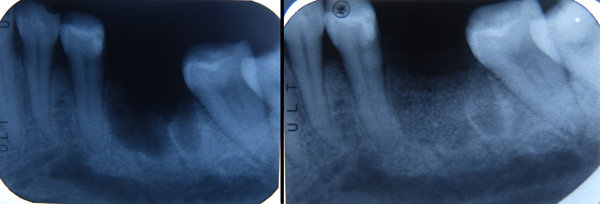
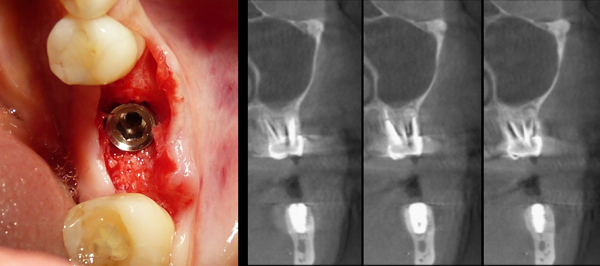
CBCT scans were performed before grafting procedures and after 6 months of healing (Fig. 7) in order to evaluate the quality and quantity of the newly formed bone that filled the defect. A clinical examination was performed during the re-entry surgery after 6 months (Fig. 8) for implant insertion procedures (Fig. 9). Implants follow-up included a radiological examinations after 4 months (Fig. 10), at final crown delivery and every 6 months.
A total of 19 titanium dental implants were inserted during the surgical re-entry (6 months).The mean follow up period was 12 months.
3. RESULTS
In all cases, after 6 months of healing, the defects were completely filled by newly formed bone. All cases showed complete bone filling by clinical and radiographs observation. Surgical data and radiological measurements for each patient are summarized in Table 1. The newly formed tissue, observed during the surgical re-entry, had a compactness similar to the medium-density bone. The presence of graft particles or grains in sub mucous connective tissues was not recorded. No graft particles or grains were visible in the regenerated bone structure that appeared homogeneous and uniform. A D2-D3 bone density was detected during implant drilling procedures. All inserted implants reached high values of primary stability. In all cases, after 18 of 19 implants were inserted, complete osseointegration after proper healing period was achieved. 1 implant failed, and non osseointegration was detected during the second implant stage surgery. After the follow-up period of 12 months, hard and soft tissues were stable. The healing of soft tissues after grafting procedures was particularly free of complications. Even in cases where the surgical wound had not been closed by first intention, complete defect coverage was observed within 10-15 days without complications or painful symptoms.


| Patient no. | Description | Notes | Tooth Used | Initial Defect (CBCT prior grafting procedures) | Defect Filling (CBCT after 6 monts) |
|---|---|---|---|---|---|
| 1 | Post extraction socket preservation. 100% autologous tooth graft Resorbable collagen membrane (pericardium). | Insertion torque 35 N | First lower left molar | Mesio - Distal: 9.69 mm Bucco – Lingual: 7.04 mm Height: 5.40 mm (Loss of lingual cortex) |
Mesio - Distal: 9.08 mm Bucco – Lingual: 9.80 Height: 4.20 mm |
| 2 | Post extraction socket preservation. 100% autologous tooth graft. Resorbable collagen membrane (pericardium). | - | Second lower second premolar | Mesio - Distal: 8.94 mm Height: 7.23 mm |
Mesio - Distal: 8.94 mm Height: 7.20 mm |
| 3 | Vertical guided bone regeneration. One implant was placed in a contiguous zone. Resorbable collagen membrane (pericardium). | - | Third upper right molar | Mesio - Distal: 11.55 mm Height: 10.05 mm |
Mesio - Distal: 11.55 mm Height: 10 mm |
| 4 | Post extraction socket preservation. 100% autologous tooth graft. Resorbable collagen membrane (pericardium). | Loss of palatal cortex | First right upper central incisor | Mesio - Distal: 11.30 mm Bucco - Lingual: 10.37 mm Height: 10.53 mm |
Mesio - Distal: 11.30 mm Bucco - Lingual: 12.22 mm Height: 10.56 mm |
| 5 | Post extraction socket preservation. 100% autologous tooth graft. Resorbable collagen membrane (pericardium). | Implant failure | First left upper central incisor | Mesio - Distal: 13.18 mm Bucco - Lingual: 14.47 mm Height: 7.79 mm |
Mesio - Distal: 13.18 mm Bucco -Lingual: 14.50 Height: 7.80 mm |
| 6 | Post extraction socket preservation. 100% autologous tooth graft. Resorbable collagen membrane (pericardium). | - | First upper left premolar | Mesio - Distal: 11.30 mm Bucco - Lingual: 9.27 mm Height: 8.23 mm |
Mesio - Distal: 11.30 mm Bucco - Lingual: 8.89 mm Height: 8 mm |
| 7 | Vertical guided bone regeneration. 100% autologous tooth graft. Resorbable collagen membrane (pericardium). | Loss of buccal cortex | First and second lower left premolar | Bucco - Lingual: 7 mm Height: 8 mm |
Bucco - Lingual: 10.03 mm Height: 8.2 mm |
| 8 | Post extraction socket preservation. 100% autologous tooth graft. Resorbable collagen membrane (pericardium). | Presense of apical cyst |
Second upper left molar | Mesio - Distal: 8.52 mm Bucco - Lingual: 10.72 mm Height: 11.7 mm |
Mesio - Distal: 9 mm Bucco - Lingual: 10.66 mm Height: 10.95 mm |
| 9 | Post extraction socket preservation. 100% autologous tooth graft. Resorbable collagen membrane (pericardium). | Loss of lingual cortex | Central lower incisors | Mesio - Distal: 12.04 mm Bucco - Lingual: 7 mm Height: 10.91 mm |
Mesio - Distal: 12 mm Bucco - Lingual: 6 mm Height: 10.34 mm |
| 10 | Post extraction socket preservation. 100% autologous tooth graft. Resorbable collagen membrane (pericardium). | Presence of apical cyst with lingual cortex loss. | Deciduos teeth preserved in a plastic box from 15 years. |
Mesio - Distal: 8.57 mm Bucco - Lingual: 4.72 mm Height: 10.10 mm |
Mesio - Distal: 8.58 mm Bucco - Lingual: 4.80 mm Height: 10.50 mm |
| 11 | Post extraction socket preservation. 100% autologous tooth graft. Resorbable collagen membrane (pericardium). | - | Second upper left molar | Mesio - Distal: 13.18 mm Bucco - Lingual: 14.47 mm Height: 8 mm |
Mesio - Distal: 13 mm Bucco - Lingual: 14.50 mm Height: 8 mm |
| 12 | Sinus lift (lateral window technique) using tooth as graft material. Readsorbable membrane to protect the sinus membrane. | Implant insertion after 6 months | Three wisdom teeth preserved in plastic box for twenty years | - | Volume maintained, uniform radiopacity |
| 13 | Sinus lift (lateral window technique) using tooth as graft material. Readsorbable membrane to protect the sinus membrane. | Immediate implant insertion | Upper right first molar | - | Volume maintained, uniform radiopacity |
| 14 | Sinus lift (lateral window technique) using tooth as graft material. Readsorbable membrane to protect the sinus membrane. | Immediate implant insertion | First and second right premolars | - | Volume maintained, uniform radiopacity |
| 15 | Sinus lift (lateral window technique) using tooth as graft material. Readsorbable membrane to protect the sinus membrane. | Presence of apical cyst | First upper right premolar and first upper right molar | - | Volume maintained, uniform radiopacity |
4. DISCUSSION
The ideal grafting material should provide scaffolds for bone regeneration (osteoconduction) and, at the same time, should promote the recruitment of bone forming cells (such as preosteoblasts) and induce new bone formation (osteoinduction) [19].
Autologous bone graft has been considered the gold standard in bone regeneration procedures but donor site morbidity, pain, and prolonged hospitalization (in case of extra oral donor sites) have prompted the search for bone graft substitutes [20]. Too fast reabsorption rate has also been reported for autologous bone graft.
Xenograft materials have been used for many years with success [21], in various fields of oral bone regeneration procedures, often in association with dental implants. Many studies [22, 23] demonstrated that these regeneration materials are space maintaining and efficient scaffolds [24] for osteogenetic cells migration but they did not offer any osteo-induction properties. The chemical or physical processes to eliminate any organic residuals, which all xenograft materials are used, destroyed all proteins that are fundamental in bone regeneration promotion. Other authors reported [25] that allografts show faster turnover and a quicker decrease in biological action than xenografts.
On the other hand, many studies demonstrated that demineralized tooth graft is able to maintain the autogenous growth factors (such as osteopontin, dentin sialoprotein and BMP) and, for this reason, could induce bone formation (osteoinduction).
In fact, dentoalveolar ankylosis with osseous replacement is often seen after replantation of avulsed teeth and this exact mechanism could explain the osteoinductive properties of demineralized dentin matrixare that acts as a slow-releasing carrier of Bone Morphogenic Proteins (BMP) [26] and it was observed that tooth graft material produced a similar amount of new bone compared to autogenous bone graft (iliac crest) [27].
Growth factors, such as Insulin-like Growth Factor (IGF), Bone Morphogenetic Protein-2 (BMP-2) and Transforming Growth Factor (TGF-β) are preserved over the time; in fact, they were extracted from archaeological compact human bone and tooth dentin dating from the late pre-ceramic pottery Neolithic and the early Middle Ages [28].
Previous authors [29] used autogenous demineralized dentin matrix on dental socket wound healing process in humans and they reported that dentin matrix gradually disappeared from the dental socket during the course of the repair process, suggesting its resorption during the bone remodeling process.
In the 2014 [30] tooth bone graft was considered as a good alternative to a heterologous bone graft when extraction is necessary prior to the surgery and two years later, in 2016, a case series was published showing successful subsistence of the cortico-cancellous bone volume using with an average follow-up of 5 years .
A recent literature review [31] identified 108 studies about autogenous teeth used for bone grafting and selected 6 among them. The authors reported an implant survival rate of 97.7% and found that the dehiscence of the wound was a frequent complication. Another animal study [32] showed accelerated bone healing in defects treated by autogenous demineralized dentin matrix and PTFE membrane in respect with PTFE membrane alone.
Clinical studies [33] on Guided Bone Regeneration (GBR), socket preservation, and ridge augmentation showed new bone formation and the crestal bone resorption during the follow-up period was very low.
Pang et al. [34], compared clinical and histological per-formances of autogenous demineralized dentin matrix from extracted tooth versus anorganic bovine bone in 33 cases of socket preservation after tooth extraction. They found new bone formation and vertical bone increase in both groups without any significant difference.
The results showed by the present study confirmed those showed by Kim YK et al., that found favorable bony healing by osteoconduction in a GBR case series study with 15 patients and a 31-month follow-up period [35].
This paper shows clinical and radiological observations of a limited number of cases in which tooth graft was used for bone regeneration procedures. Standardized and controlled studies with large sample size are needed, in the next future, to validate the findings of the present case series analysis.
CONCLUSION
This innovative device allowed to process and use, as bone graft, any patient’s tooth in a very short time. All decontamination, disinfection and demineralization processes are totally electronically managed by the machine itself without any possibility of human error or injury.
Future studies with long follow up period are needed in order to better evaluate the potential of demineralized dentin autografts.
ETHICS APPROVAL AND CONSENT TO PART-ICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies that are the basis of this study.
CONSENT FOR PUBLICATION
An informed and written consent to the surgical treatment was obtained from each patient.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Marco Berardini and Paolo Trisi claim to have no financial interest, either directly or indirectly, in the products or information listed in the article. Elio Minetti acts as consultant for TT TOOTH TRANSFORMER SRL (Via Washington, 59 - Milan, Italy).


