All published articles of this journal are available on ScienceDirect.
Association of Periodontal Disease and Polycystic Ovarian Syndrome: A Systematic Review and Meta-analysis with Trial Sequential Analysis
Abstract
Introduction:
Several articles have suggested a potential synergistic relationship between periodontal disease and systemic inflammatory diseases, such as Polycystic Ovarian Syndrome (PCOS) and diabetes mellitus. However, the associations between periodontal disease and PCOS population remain unclear in the literature.
Objective:
The primary aim of this review is to examine the associations between periodontal disease and PCOS with different scoring methods, namely clinical attachment loss, probing depth, gingival index, percentage of bleeding on probing and plaque index.
Methods:
MEDLINE, EMBASE and CENTRAL were systematically searched for observational studies and case-control studies from its inception until 2nd June, 2019. Case reports, case series, non-systematic reviews and trials published as abstracts were excluded.
Results:
Four articles (614 subjects) were included for analysis. Out of 614 subjects, 329 PCOS patients were compared to 285 healthy subjects. In comparison to healthy cohort, women with PCOS had a statistically significant increase in clinical attachment loss (MD: 0.34, 95% CI: 0.13-0.55, ρ=0.002), probing depth (MD: 0.35, 95%CI: 0.21-0.48, ρ<0.001), gingival index (MD: 0.70, 95% CI: 0.70-1.11, ρ<0.001) and percentage of bleeding on probing (MD: 34.41, 95% CI: 20.23-48.59, ρ<0.001). No difference was demonstrated in plaque index (MD: 0.42, 95% CI: -0.29-1.12, ρ=0.24) for both PCOS and healthy cohort.
Conclusion:
PCOS is significantly associated with a higher severity of the periodontal disease. This association should be emphasized during the management of PCOS patients, by including referral to dentists or periodontists for regular mechanical debridement of plaque and periodontal maintenance.
1. INTRODUCTION
Periodontitis, a leading cause of tooth loss in adults, is a complex, multifactorial and inflammatory disease of the supporting structures of the teeth, characterized by an exaggerated gingival inflammatory response, causing alveolar bone loss and tooth loss if left untreated [1]. The destruction of the periodontal tissue is mainly due to an inflammatory host response secondary to infection by periodontal bacteria; however, it is also influenced by genetic, systemic and environmental factors [2]. Periodontitis is the second most prevalent oral disease globally, and the sixth most prevalent disease on the Global Disease Burden list [3], predicted to remain constant from 1990 to 2020 [4].
Current literature suggests an association between periodontitis and systemic conditions such as cardiovascular disease [5, 6], diabetes mellitus [7], metabolic syndrome [8] and preterm low birth weight [9-11]. Periodontal disease is associated with systemic inflammation [12-16]. In this context, there may be a bi-directional relationship between periodontal disease and Polycystic Ovarian Syndrome (PCOS) which is associated with low-grade systemic inflammation [17]. PCOS is characterized by menstrual irregularities, chronic anovulation and hyperandrogenism. It is the most prevalent endocrinopathy in women of reproductive age, with a global prevalence ranging from 6% to 15% [18].
PCOS patients are at a higher risk of developing Insulin Resistance (IR) and hyperinsulinemia, believed to induce low-grade chronic systemic inflammation affecting oral health [19-21]. Several articles have suggested a potential synergistic relationship between periodontal disease and PCOS [22-25]. However, the association between periodontal disease and the PCOS population remains unclear in the literature. Several studies reported a strong association between periodontal disease and PCOS with a high level of systemic inflammatory markers, including adhesion molecules, TNF-α, IL-1β, IL-6 and monocyte chemoattractant protein 1 [26-28].
We hypothesized that patients diagnosed with PCOS are associated with higher severity of the periodontal disease. The primary aim of this systematic review and meta-analysis was to examine the association between periodontal disease and PCOS with different scoring methods, namely Clinical Attachment Loss (CAL), probing depth, gingival index, percentage of bleeding on probing and the plaque index.
2. METHODS
This review paper was conducted according to the `Preferred Reporting Items for Systematic Review and Meta-analysis' statement. The review protocol was registered on PROSPERO (CRD42018110584). The research questions were formulated using a Participant, Intervention, Comparison and Outcomes (PICO) model (S-Table 1) Online Supplementary Material). The primary outcome was clinical attachment loss. Secondary outcomes were probing depth, percentage of bleeding on probing, plaque index and gingival index.
Ovid MEDLINE, EMBASE and CENTRAL were systematically searched from its inception until 2nd June, 2019 (S-Table 2). Trial registers (ClinicalTrials.gov and World Health Organisation International Clinical Trials Registry Platform) were searched to identify any unpublished or ongoing studies. The inclusion criteria were adult women diagnosed with PCOS (≥18 years old) and non-obese with a BMI <30kg/m2, who were matched with a healthy cohort. The diagnosis of PCOS was made based on the 2003 Rotterdam criteria. All observational studies and case-control studies that examined the association of periodontal disease and PCOS and a healthy cohort were identified in this review, regardless of the measured outcomes. Case reports, case series, non-systematic reviews and trials published as an abstract were excluded. Articles not written in the English language were included if the journal provided an English-translated version. All the bibliographies of included papers were hand-searched for additional papers (Table 1). Authors of the included studies were contacted at least twice if any data were missing.
Titles and abstracts were independently screened against eligibility criteria by two authors (KN and MN). If both authors were confident that a study was unsuitable, based on the titles and abstracts, this study was excluded. Any disagreements at this stage were resolved by the third author (FF). The same two reviewers (KN and MN) independently screened full texts of qualifying papers. Any discrepancies at this stage were discussed with the third author (FF) to achieve a final decision.
All the studies included were assessed for risk of bias using the Newcastle-Ottawa Scale (Table 2). The GRADE assessments of the quality of evidence (Table 3) and summary of findings were independently performed by two authors (KN and MN). Any disagreements were resolved by the third author (FF). Based on the Cochrane Handbook, the quality of evidence was assessed based on the five criteria (risk of bias, inconsistency, indirectness, imprecision and publication bias).
Statistical analyses were done using the RevMan Review Manager Version 5.3. Analysis of funnel plots was not performed, as there were less than 10 studies in each of the measured outcomes. The I2 test was used to assess the heterogeneity of studies. The values of <40%, 40-60% and >60% were used to determine low, moderate and substantial heterogeneity, respectively. A two-sided ρ-value of <0.05 was considered to denote the statistical significance of heterogeneity. All findings were reported as Odds Ratios (OR) or Mean Difference (MD) with 95% (CI). A fixed-effect model analysis (Mantel-Haenszel method) was used. If evidence of substantial heterogeneity (I2>60%) was observed, a random-effects model analysis (DerSimonian–Laird method) was used. To investigate the presence of small-study effects on all the measured outcomes, sensitivity analysis with both fixed and random effect models was compared to detect any changes in the magnitude and direction of statistical findings. In addition, sensitivity analyses were performed by sequentially removing each study (from the most recent trials) and re-analyzing the remaining dataset of those outcomes with substantial heterogeneity.
Using the trial sequential analysis viewer version 0.9.5.5 Beta, a trial sequential analysis was performed on all the measured outcomes to prevent the risk of random error and the multiplicity phenomenon due to repeated significance testing in meta-analyses. The required meta-analysis information size and adjusted significance thresholds were derived based on a two-sided sequential analysis-adjusted fixed/random effects model with 5% risk of type 1 error and power of 80%.
3. RESULTS
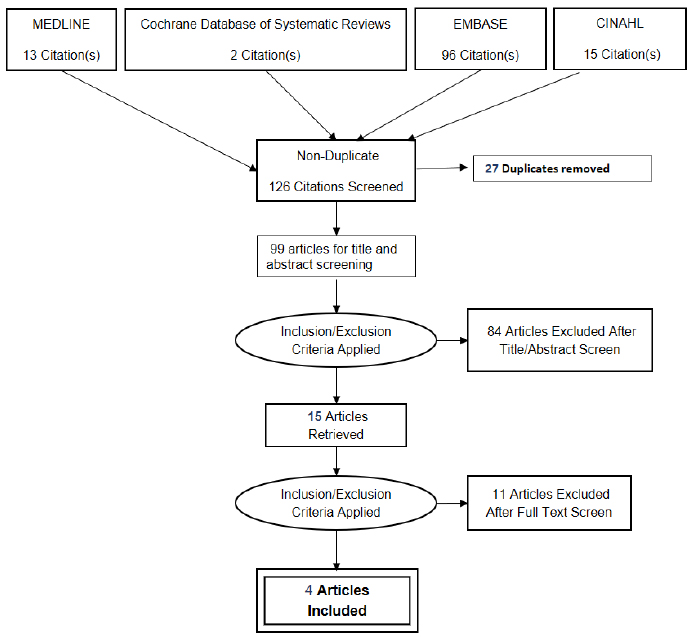
The comprehensive literature search yielded 126 citations. Of the 99 citations screened for the title and abstract, 15 articles were retrieved for full-text screening. After the full-text screening, four articles (614 subjects) were included in this review (Fig. 1) All the characteristics of the excluded articles are illustrated in S-Table 3 (Online Supplement).
The clinical characteristics of the studies included are displayed in Table 1. The four studies were observational, prospective single-centre, and case-controlled in nature. All were conducted in Asian countries. Out of 614 subjects, 329 PCOS patients were compared to 285 healthy controls to examine the association between periodontal disease and PCOS. The mean age and body mass index of the PCOS group were 24.76 ± 5.96 years and 26.62 ± 7.60 kg/m2, respectively; the mean age and body mass index of the healthy controls were 25.36 ± 5.41 years and 22.84 ± 3.91 kg/m2, respectively. No potential conflict of interest was declared in the four case-control studies. In terms of the risk of biased assessment, all studies were graded as low risk (S-Table 4 Online Supplement). The summary of findings with quality of evidence (GRADE) assessment and PRISMA checklist is demonstrated in S-Table 5 and S-Table 6 respectively (Online Supplement).
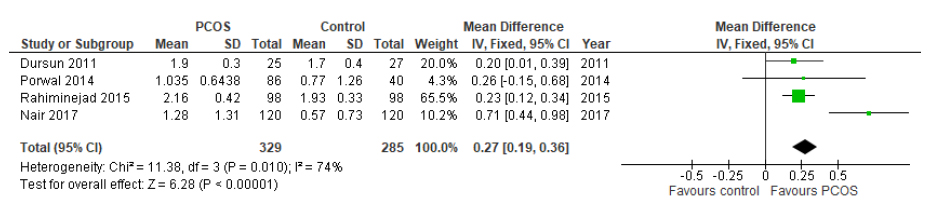
Based on the data of the studies included (four studies,n=614 subjects), there was a statistically significant increase in clinical attachment loss in women with PCOS compared with women without PCOS (MD 0.27, 95%CI: 0.19-0.36, p˂0.00001, (Fig. 2). The statistical heterogeneity was high across all studies (I2=74%). At this stage, with 614 subjects, only 40.3% of the required information size (1,522 patients) was achieved to detect a significant effect of the measured outcomes (Fig. 3)
There was a statistically significant increase in probing depth in women with PCOS compared with women without PCOS (two studies, n=178 subjects; MD 0.35, 95% CI: 0.21-0.48, ρ<0.001, S-Fig. (1)). The statistical heterogeneity was low (I2=5%). In the trial sequential analysis, our current review achieved the minimum required information size of 114 and it is conclusive that the probing depth was deeper in the PCOS cohort than the healthy cohort S-Fig. (2)
In terms of the gingival index, the PCOS cohort had a statistically significantly higher value than the healthy cohort (three studies, n=418 subjects; MD 0.70, 95% CI: 0.29-1.11, ρ< 0.001, S-Fig. (3)). The heterogeneity across all studies was substantial (I2=89%). The trial sequential analysis of this outcome is conclusive that the PCOS cohort had a more severe gingival index than the healthy cohort S-Fig. (4)
There was a statistically significant increase in the percentage of bleeding on probing in the PCOS cohort, in comparison to the healthy cohort (two studies, n=178 subjects; MD 34.41, 95% CI: 20.23- 48.59, ρ<0.001, S-Fig. (5). The heterogeneity of the included studies was low (I2=34%). In the trial sequential analysis, the current data has exceeded the required information size of 134 patients and the z-curve (blue line) crossed the boundary line of benefit favouring control group, indicating that the PCOS cohort had a higher percentage of bleeding on probing than the healthy cohort S-Fig. (6).
There was no statistically significant difference in the plaque index between women with PCOS compared to women without PCOS (three studies, n=374 subjects; MD 0.42, 95% CI: -0.29-1.12, ρ=0.24, S-Fig. (7)). The statistical test of heterogeneity was significant. The required information size for the plaque index was not calculated due to limited information. In the law of iterated logarithm, the penalised z-curve (green line) did not cross the boundary of benefit, indicating that it is inconclusive that the PCOS cohort was associated with a higher degree of plaque index (S-Fig. 8).
Sensitivity analyses were performed on the primary and secondary outcomes and the estimated effects remained unchanged, except for the percentage of bleeding on probing and the plaque index. By sequentially removing the most recent trials and re-analysing the remaining dataset, it detected major changes in the magnitude of statistical findings for the percentage of bleeding on probing and plaque index, which indicated the potential evidence of between-study heterogeneity among the included studies.
4. DISCUSSION
To the best of our knowledge, this is the first meta-analysis demonstrating the association between PCOS and periodontal disease. We conducted an exhaustive literature search and the chosen studies had a rigorous methodological assessment. We also standardized the diagnosis of PCOS based on the Rotterdam criteria 2003, as part of our inclusion criteria, to minimize the degree of heterogeneity [29].
Our meta-analysis showed that the PCOS cohort, in contrast to the healthy cohort, was significantly associated with periodontal disease, in terms of clinical attachment loss, probing depth, gingival index and percentage of bleeding on probing and there was no statistical significance for the plaque index. However, the quality of the evidence for all the measured outcomes was very low due to the high degree of inconsistency and imprecision.
From this meta-analysis, the PCOS cohort had a higher risk of developing severe periodontal disease. Several pathophysiological links between PCOS and periodontal disease have been suggested in literature including low-grade systemic inflammation, Insulin Resistance (IR), oxidative stress, advanced glycation end products as well as systemic hormonal levels [30]. Evidence has shown that chronic subclinical inflammation could be caused by periodontal disease leading to IR, initiating the development of type 2 diabetes, a prominent feature in PCOS [31-33]. It is also hypothesised that the sexual hormonal changes (hyperandrogenism, hyperestrogenism and hyperprogesteronism) in PCOS induce chronic low-grade inflammation affecting the capillary system and the angiogenesis process. Subsequently, it disrupts the defence system of periodontal tissues to the microbial plaque by altering the proliferation of oral flora and inducing pro-inflammatory cytokines [21, 34, 35].
| Study (Year) | Location | Design | Study Population | N | Confounders Assessed | Age | BMI | Clinical Outcomes |
|---|---|---|---|---|---|---|---|---|
| Dursun 2011 |
Turkey | Case-control study | Non obese never smoker women with normal glucose tolerance and without history of systemic disease or any drug use (oral contraceptives for ≥ 3 months) S: PCOS diagnosed with Rotterdam Criteria (n=25) C: Age and weight matched systemically healthy women with regular menstrual cycles without clinical or biochemical hyperandrogenism or PCOS (n=27) |
52 | Cushing's syndrome, congenital adrenal hyperplasia, hyperprolactinemia, thyroid disorders, androgen secreting tumors, smoking, oral contraceptives use ≥3 months. BMI | S: 22.7±3.6 C: 24.2±2.5 |
S:22.4±2.50 C: 20.6±1.9 |
CAL,PD,GI,%BOP,PI |
| Porwal 2014 |
India | Case-control study | Reproductive age women diagnosed with >16 natural teeth. S: PCOS diagnosed with Rotterdam Criteria (nN=41, nM=45) C: systemically healthy age matched regularly menstruating women with no clinical or biochemical sign of hyperandrogenism and ultrasound exclusion of PCOS (n=40) |
126 | Thyroid disorders, hyperprolactinemia, androgen-secreting tumors, chronic inflammatory diseases (nephrotic syndrome, chronic renal failure, significant cardiovascular disease, established type 1 or type 2 diabetes mellitus, or active cancer within the past 5 years), smoking, alcoholism, antibiotics within 3 months, periodontal treatment within 6 months, aggressive periodontitis and BMI | N:23.09±4.90M:22.68±4.55 C:23.50±2.67 |
N: 25.01±3.61 M: 24.57±4.38 C: 23.76±4.82 |
CAL,PD,GI,%BOP,PI |
| Rahiminejad 2015 |
Iran | Case-control study | 18-45 years of age women with BMI<25 kg/m2 and without IGT in early follicular phase S: PCOS diagnosed with Rotterdam Criteria (n=98) C: systemically healthy women (n=98) |
196 | Pregnancy, smoking, malignancies, osteoporosis, BMI, IGT, thyroid diseases, hyperprolactinemia, Cushing’s syndrome, androgenic tumors, 21-hydroxylase deficiency, periodontal treatment or prophylactic antibiotic therapy during the past 6 months and hormonal effect of menstrual cycle | S:29.06±6.56 C:28.60±6.37 |
S: 23.81±4.10 C: 24.02±3.50 |
CAL,PD,%BOP, PI, tooth loss |
| Nair 2017 |
India | Case-control study | 18-45 years of age women having minimum of 20 teeth in early follicular phase S: PCOS diagnosed with Rotterdam Criteria (n=120) C: systemically healthy women (n=120) |
240 | Pregnancy, smoking, alcoholism, malignancy, osteoporosis, periodontal treatment or prophylactic antibiotic therapy during the past 6 months and hormonal effect of menstrual cycle | S: 23.40±4.47 C:23.6 ± 4.46 |
S: 24.39 ± 4.52 C: 22.09 ± 3.81 |
CAL,GI |
| No | Reference | Case-cohort Representative | Selection of Non-exposed Control | Ascertainment of Exposure | Outcome Negative at Start | Comparability by Design | Comparability by Analysis | Outcome Assessment | Duration of Follow-up | Score |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Dursun et al | * | * | * | * | * | * | * | * | 8 |
| 2 | Porwal et al | ** | * | * | * | * | * | * | * | 9 |
| 3 | Rahiminejad et al |
* | * | * | * | * | * | * | * | 8 |
| 4 | Nair et al | x | * | * | * | * | * | * | * | 7 |

| Certainty Assessment | № of Patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| № of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | PCOS | Healthy Cohort |
Relative (95% CI) |
Absolute (95% CI) |
||
| Clinical Attachment Loss | ||||||||||||
| 4 | observational studies | not serious | very serious a | not serious | not serious | none | 329 cases 285 controls |
RR 0.27 (0.19 to 0.36) |
- |
|
– | |
| - | 0.0% |
0 fewer per 1,000 (from 0 fewer to 0 fewer) |
||||||||||
| Probing depth | ||||||||||||
| 2 | observational studies | not serious | not serious | not serious | serious b | none | 111 cases 67 controls |
RR 0.35 (0.21 to 0.48) |
- |
|
– | |
| - | 0.0% |
0 fewer per 1,000 (from 0 fewer to 0 fewer) |
||||||||||
| Gingiva Index | ||||||||||||
| 3 | observational studies | not serious | very serious a | not serious | not serious | none | 231 cases 187 controls |
RR 0.70 (0.29 to 1.11) |
- |
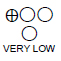
|
– | |
| - | 0.0% |
0 fewer per 1,000 (from 0 fewer to 0 fewer) |
||||||||||
| Bleeding on Probing (%) | ||||||||||||
| 2 | observational studies | not serious | very serious a | not serious | serious b | none | 0 cases 0 controls |
RR 34.41 (20.23 to 48.59) |
- |
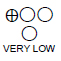
|
– | |
| - | 0.0% |
0 fewer per 1,000 (from 0 fewer to 0 fewer) |
||||||||||
| Plaque index | ||||||||||||
| 3 | observational studies | not serious | very serious a | not serious | serious b | none | 209 cases 165 controls |
RR 0.42 (-0.29 to 1.12) |
- |
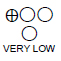
|
– | |
| - | 0.0% |
0 fewer per 1,000 (from 0 fewer to 0 fewer) |
||||||||||
Explanations
a. Substantial heterogeneity
b. Total participants <400
However, there are many confounding factors, which were unadjusted or uncontrolled due to the nature of observational studies. First, the diagnosis of PCOS is complex, with different definitions in the literature [33]. In this review, we used the Rotterdam criteria, a combination of clinical, biological and ultrasound evaluation to standardize the diagnosis of PCOS in all four studies [29]. Finding polycystic ovaries in healthy women with an ultrasound occurs frequently, and this may overestimate the diagnosis of PCOS [36]. In addition, the pathophysiology of PCOS is multifactorial, involving a combination of endocrine (hyperglycemia, dyslipidemia) and metabolic (obesity) components with environmental factors (smoking, stress and physical activity) [37]. It may contribute to the significant heterogeneity of our findings. This review provided only a positive association of PCOS and periodontal disease, not a causal relationship. Our findings correspond with a review performed by Kellesarian and colleagues [38]. However, the review included several studies with selection bias and duplicates of sample size, which may introduce potential bias in their findings. A scoping review conducted by Arbildo and the team showed a positive and significant association between PCOS and the clinical or molecular parameters of periodontal diseases [39]. The finding has to be interpreted with a caveat due to the nature of a non-systematic search. The review has missed out on the study by Nair et al, 2017 [40] and included several studies with selection bias.
In this review, the PCOS cohort was associated with a significantly higher degree of Clinical Attachment Loss (CAL) and probing depth compared to the healthy cohort. Clinical attachment loss is the best clinical index as it relies on a fixed reference point, the cementoenamel junction, to determine periodontitis and the longitudinal measurement of CAL is considered the gold standard for recording changes in periodontal status [41]. The probing pocket depth, however, can be influenced by the changes at the position of the gingival margin since it is measured from the gingival margin to the base of the pocket [41]. This finding has to be interpreted with caution due to the high level of heterogeneity and inconclusive trial sequential analysis of clinical attachment loss. At the current review level, only 40.3% of the required information size of 1,522 patients was achieved to detect a significant effect of clinical attachment loss. In addition, the severity of the periodontal disease can be influenced by several confounding factors, mainly mechanical debridement of plaque, glycemic control and smoking [22, 25]. In the current study, all four studies excluded smokers. Except for Dursun et al., 2011, all other studies reported the exclusion of patients who had periodontal treatment in the last six months and Nair et al., 2017, did not report the exclusion of glycaemic control.
Porwal et al., 2014, reported a negative association between periodontal diseases and PCOS patients who received medical treatment, compared to the group without medical treatment.
The concomitant metabolic syndrome with PCOS may potentially cause or worsen periodontal disease. Obese patients with a BMI > 30m/kg2 were part of our exclusion criteria in this review. However, two reviews suggested that waist circumference and waist-hip ratio are superior to BMI in the measurement of central adiposity. Hung et al., 2017, suggested using both BMI and analysis of biometric impedance in the diagnosis of obesity and overweight in young Asian adults [42].
Gingivitis often precedes the occurrence of periodontitis [43]. It is reversible if detected early [43]. Various indices have been proposed with different methodologies, but none has universal acceptance or application [44]. The gingival index developed by Loe and Silness in 1963, has been used frequently in clinical trials of therapeutic agents. It has good sensitivity and reproducibility, with the stipulation that the examiner's knowledge of periodontal biology and pathology is optimal [43]. In comparison to the healthy cohort, the PCOS cohort had significantly severe gingivitis in terms of the gingival index and the percentage of bleeding on probing.
We reported no significant differences in the plaque index between the PCOS cohort and the healthy cohort. Although the plaque index was not statistically significant, our review showed that the PCOS cohort had a higher plaque index than the healthy cohort. Certain factors, such as the presence of specific sub-gingival bacteria, cigarette use, diabetes and age, increase the risk of plaque-induced gingivitis and chronic periodontitis [45]. These confounding factors may contribute significant heterogeneity to the outcome of the plaque index.
Within the limits of the available evidence, there appears to be a positive relationship between PCOS and periodontal disease. The awareness of the association between periodontitis and such systemic conditions emphasizes the need for communication between the dental and medical professionals [46]. Future investigations are necessary in order to conclude a causal association and interrelationship between PCOS and periodontal disease to increase understanding in this field.
CONCLUSION
Our meta-analysis demonstrated that women diagnosed with PCOS showed a significantly higher association with periodontal disease than the healthy cohort within the limits of the available evidence. The significance of this association should be emphasized during the management of PCOS patients, by referring the patients to dentists or periodontists for regular mechanical debridement of plaque and periodontal maintenance. Such dental treatment will prevent the risk of developing periodontal disease and subsequent dentition loss in patients with PCOS.
CONSENT FOR PUBLICATION
Not applicable.
STANDARD OF REPORTING
We followed the PRISMA guidelines and registered with PROSPERO ID: CRD42018110584.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
We would like to acknowledge Dr Susanna Wright of King Abdullah International Medical Research Center for her assistance in editing the manuscript.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publishers Website along with the published article.


