All published articles of this journal are available on ScienceDirect.
Sealability of Different Root Canal Nanosealers: Nano Calcium Hydroxide and Nano Bioactive Glass
Abstract
Background:
The success of the endodontic treatment is largely dependent on the sealing achieved by root canal obturation. The application of sealer fills imperfections and increases adaptation of the root filling to the canal walls.
Aim:
To evaluate the sealability of experimental nanosealers (nano calcium hydroxide and nano bioactive glass) and to compare it with the commercial zinc oxide eugenol sealer using a dye penetration method.
Materials and Methods:
Sixty single-rooted mandibular premolars were selected. The tooth crowns were removed so as to obtain standardized 15-mm-long root specimens. The root canal was instrumented with Protaper Ni-Ti rotary file and the final file size was up to # F4/.06 (in vitro study). They were then randomly allocated into 3 groups of 20 specimens each (n=20) according to the sealer used for obturation, and all samples were filled with single cone gutta-percha (#40/06) and one of the tested sealers. All teeth were coated with nail polish and then suspended in 2% methylene blue dye for 7 days. Stereo-microscope (x10) was used to evaluate the sealability of newly introduced nanosealers. The data were statistically analyzed by ANOVA test followed by post hoc analysis (P < 0.05).
Results:
Significant improvement shown by the presented study suggests that nano calcium hydroxide sealer showed significantly less dye leakage than nano bioactive glass sealer and zinc oxide eugenol sealer.
Conclusion:
The results of this study showed that the synthesized nano-powder sealers are suitable for use in root canal therapy to prevent leakage.
The root canal can be sealed better by using smaller nano-powder particle sizes. In addition, the two groups exhibited significant differences in leakage in comparison with commonly used ZOE sealer.
1. INTRODUCTION
Adequate obturation of the root canal system constitutes one of the most important stages of endodontic treatment, which significantly affects its final result [1]. Therefore, a permanent three-dimensional seal is developed from the apical foramen to the root canal orifice.
A variety of materials are available for root canal obturation; however, the Gutta-percha cones along with the sealer remains the most accepted choice of the clinician.
The application of sealer fills imperfections and increases adaptation of the root filling to the canal walls. Different types of sealers have been used in conjunction with Gutta-percha for root canal obturation with varied success [2-4].
It has been documented that teeth obturated with Gutta-percha along with sealer display a better sealing ability than those obturated without sealer [5-7]. Incomplete sealing and presence of spaces between the root canal wall and the obturating material can lead to failure in apparently good treatment [8, 9]. To overcome these drawbacks, new nanoparticles sealers have been introduced to enhance the sealability [10].
Nanotechnology is the science of evaluating and producing materials in nano-dimensions by re-location and re-arrangement of atoms to prepare materials with better properties. Nanoparticles (Np) are ultrafine particles of insoluble constituents with a diameter of less than 100 nm [11-13].
Presence of very small particles leads to superior properties of the material [14]. These unique properties, which are the subject of quantum mechanics, have attracted a great deal of interest [15]. Nanotechnology infuses scientific branches from biology, chemistry, physics, and engineering, hence called interdisciplinary subject opens new doors of applications [16].
Nanotechnology has been introduced in the dental field firstly for restorative materials [17]. Dental nanocomposites provided a cosmetically acceptable result with excellent mechanical properties for both bulk [18] and fiber-reinforced materials [19].
The use of nanofillers has also been described for endodontic sealing purposes (Cytotoxicity of Two Experimental Epoxy Resin-Based Sealers [20] and their sealing ability is an interesting variable to be evaluated.
Because of these valuable properties, utilization of nanoparticles in production of endodontic sealers has become favorable for many researchers. Recently, the authors of this article prepared new experimental endodontic sealers [nano- powder (calcium hydroxide and bioactive glass)] in the Dental Material Research Center (NanoTech Egypt for Photo-Electronics, Al-Giza, Egypt). These sealers are similar to various calcium hydroxide and bioactive glass-based sealers, but with different sizes of nano-particles. The sealing ability of our synthesized nano-sized calcium hydroxide and bioactive glass sealers was compared with zinc oxide eugenol sealer.
The recently-introduced nanosealers (nano calcium hydroxide and nano bioactive glass) are known to possess great ability to inhibit biofilm formation within the sealer dentin interface, reduce cytotoxicity, and improve sealability [21, 22].
In this case, the small size of the nanoparticles can also prove to be beneficial. Although recent research has shown that the addition of nanoparticles to the endodontic sealers does not change its physical or mechanical properties, a slight change in the flowability of the sealer was noted [23]. This can result in improving the penetration of this modified sealer into the minute dentinal tubules and better sealability.
2. MATERIALS AND METHODS (In vitro study)
2.1. Nano Calcium Hydroxide
2.1.1. Manufacture Method in this Study
(NanoTech Egypt for Photo-Electronics, Al-Giza, Egypt).
Homogeneous phase precipitation which normally takes place in the aqueous phase following mixing of a strong base (e.g., NaOH) and Ca salt (e.g., CaCl2 or Ca(NO3)2) solutions [24, 25]. Variations to this route include synthesis in organic liquids (hydrocarbons) using surfactants that adopt a reverse-type micelle configuration around Ca(OH)2 colloidal particles [26] and synthesis in water-in-oil emulsions [27].
2.2. Nano Bioactive Glass
2.2.1. Manufacture Method used in this Study (NanoTech Egypt for Photo-Electronics, Al-Giza, Egypt)
The production of 45S5 bioactive glass has traditionally been carried out through sol-gel methods. The sol-gel method is a wet-chemical technique that can be used for the fabrication of both glassy and ceramic materials [29]. In the sol-gel process, the precursors are catalyzed and dissolved in the solvent to form a sol. The sol gradually becomes a gel-like diphasic system containing both a liquid and a solid phase.
The morphology ranges from dispersed particles to continuous polymer-like networks, which mainly depends on the pH and solvent. Generally, the sol-gel process includes hydrolysis, polycondensation, drying, and stabilization. To strengthen the gelation and aging network, an aging process is necessary.
Removal of the remaining liquid solvent phase requires a drying process. Finally, a thermal treatment (stabilization) is often carried out to enhance the mechanical properties and to improve structural stability [30].
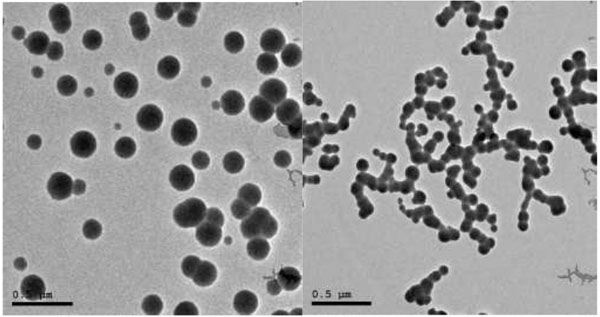
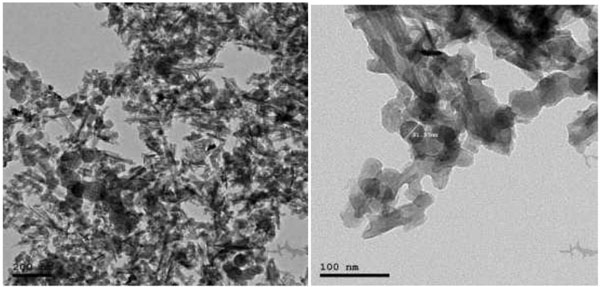
2.2.2. Size & Shape
TEM was performed on JEOL JEM-2100 high-resolution transmission electron microscope at an accelerating voltage of 200 kV, respectively (Fig. 2).
2.3. Sample Collection
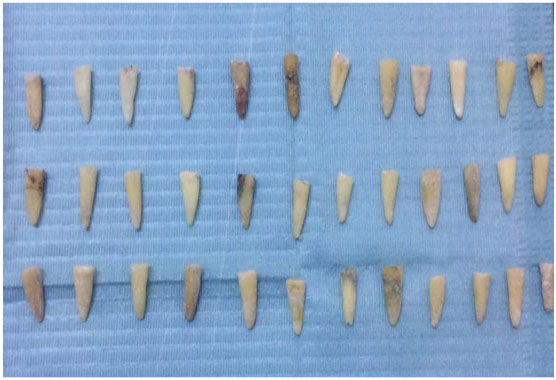
In this experimental study, freshly extracted mandibular premolars were collected with the courtesy of the oral surgery department in the faculty of dentistry Assuit University. Sixty (60) teeth were selected for the study discarding the teeth having open apices, cracks, and resorptive or morphological defects. The teeth were washed thoroughly in running tap water to remove blood and saliva.
They were rendered free of calculus and debris using hand scalers. To avoid dehydration and consequent brittleness, the samples were stored in chloramine-T solution for 24 h followed by normal saline till used in the study within 3 months [31]. (Figs. 3, 4).

2.4. Root Canal Preparation
Selected teeth were sectioned at the cementoenamel junction using a water-cooled diamond disc (Dica, Dendia, USA). The working length for all teeth was determined to obtain a standardized root length of 15mm. The root canals were instrumented with Protaper Ni-Ti (Dentsply-Maillefer, Ballaigues, Switzerland) rotary instruments up to MAF# F4/.06.
Between each instrument, the root canals were irrigated with 2.5% sodium hypochlorite (Clorox Co., 10th of Ramadan, Egypt) and physiologic saline alternatively using a long 27 gauge needle (Jiangsu Jichun Medical Devices Co., China). After the use of the last file, the roots were irrigated with physiological saline and dried with paper points, and thereafter, canals were irrigated with 5 mL of 17% Ethylene Diamine Tetraacetic Acid (EDTA) (Glyde File Prep, DENTSPLY, Ballaigues, Switzerland) for 1 min as a rinse to remove the smear layer.
Thereafter, the roots were dried with corresponding paper points [32]. This protocol was employed for all the teeth.
2.5. Obturation
After completion of the biomechanical preparation of root canal, samples were obturated using the single cone technique. We took the best samples for the study and divided them into 3 groups. Each group had 20 (n = 20) teeth by using SEM to ignore the fault samples. Group A: Canals were obturated with gutta-percha #40/.06 along with ZnoE sealer (Endofil - PROMEDICA – Germany). Group B: Canals were obturated with Guttapercha #40/.06 along with nano calcium hydroxide sealer (NanoTech Egypt for Photo-Electronics, Al-Giza, Egypt). Group C: Canals were obturated with gutta-percha #40/.06 along with nano bioactive glass sealer (NanoTech Egypt for Photo-Electronics, Al-Giza, Egypt).
2.6. Microleakage Evaluation
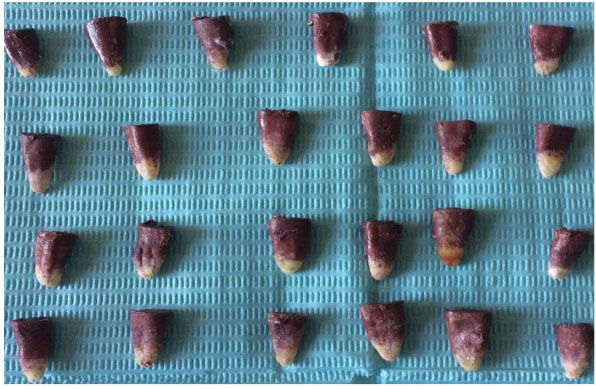
Micro leakage was estimated by dye penetration test. Teeth under the experimental group were coated with two layers of nail varnish except for the last 2mm area around the apical foramen. Specimens were then immersed in 2% methylene blue solution for 7 days at room temperature.
After being removed from the dye solution (Fig. 5), the specimens were washed and the nail varnish was scraped away from the root surface. The roots were then sectioned buccolingually in a longitudinal direction with a diamond disc under running water (Fig. 6).
The dye penetration was measured for each sample from the apex to the most coronal extent of the dye penetration under a stereomicroscope at ×10 magnification with a digital vernier caliper.
Statistical analysis of the data recorded was performed using SPSS (Statistical Package for Social Science) computer program (version 19 windows, SPSS, Egypt). One-way ANOVA test was used followed by post hoc test. Statistical program for the microleakage values with P < 0.05 was considered significant.
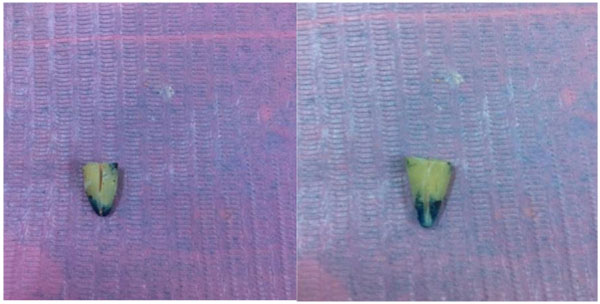
3. RESULTS
3.1. Dye Penetration Test: B
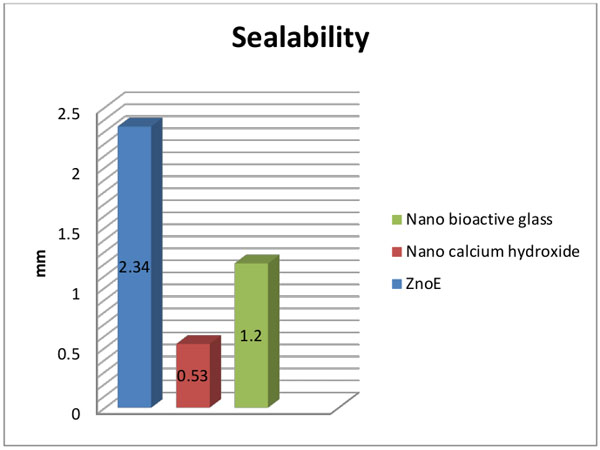
Comparison between ZnoE, nano Ca(OH)2 and nano bioactive glass sealers: Table (1), Fig. (7):
The mean and standard deviation values of sealability by dye penetration test were 2.34±0.59, 0.53±0.28, and1.2±0.84 after using ZnoE, nano Ca(OH)2, and nano bioactive glass sealers, respectively.
Nano Ca(OH)2 showed statistically significantly higher mean sealing ability (lower mean vertical apical leakage) than ZnoE and nano bioactive glass sealers (p<0.05).
3.2. Statistical Tests:
| - | ZnoE | Nano Ca(OH)2 |
Nano Bioactive Glass |
|---|---|---|---|
| N=20 | N=20 | N=20 | |
| Seal ability Range Mean ± SD |
(1.64-2.91) 2.34±0.59 |
(0.24-1.03) 0.53±0.28 |
(0.32-2.43) 1.2±0.84 |
4. DISCUSSION
The main purpose of endodontic therapy is to achieve a complete hermetic seal of the root canal and prevention of coronal and apical microleakage. Sealing ability is a crucial characteristic for endodontic materials, as even if endodontic space is isolated from the oral environment with restorations, the presence of a microleakage for restorations below the enamel cement junction [33] or V class cavities [34] has been demonstrated.
Thus, the present study aimed to evaluate the microleakage from the apical to coronal third by using recently introduced nano Ca(OH)2 and nano bioactive glass in comparison with traditional ZnoE sealer by using dye penetration test with methylene blue dye.
The appearance of nanotechnology, which is the study of the controlling of matter on an atomic and molecular scale, improves the properties and sealability of endodontic materials used as the active nanoparticles can penetrate the dentinal tubules and enter the accessory canals to ensure that the spaces have been sealed effectively [35].
Some of the advantages of using nanoparticles in endodontic sealers include improving their physicochemical characteristics, enhancing the antibacterial property, decreasing microleakage, and increasing biocompatibility [36].
To the best of our knowledge, the use of nanostructured materials as sealers in root canal therapy is limited to two or three types of nanostructured hydroxyapatite alone or in combination with epoxy resin (Nanoseal) [37]. Properties like antimicrobial activity, radiopacity, flow, film thickness, and cytotoxicity have been evaluated in various studies. We could not find any published reports on the sealing ability of nanomaterials as sealers in root canal therapy to make comparisons.
Nanoparticulate calcium hydroxide sealer is similar to various calcium hydroxide based sealers but it has been recently manufactured to eliminate the shortcomings of conventional calcium hydroxide, reducing the size of calcium hydroxide particles into nanoparticles enhances the penetration of this medicament into dentinal tubules and increases their antimicrobial efficacy [38, 39].
Bioactive-glass (B-G) has become a valuable adjunct to promote hard tissue healing in many clinical situations and is of particular interest for endodontic care because of its biocompatibility, regenerative and antimicrobial properties as well as chemical composition that closely resembles the mineral make-up of human bone and dentine. Nanoparticles of bioactive glass are a modification which enhances all these properties [40].
The dye penetration test is the most popular, probably because it is a simple and reliable method [41]. In this study, methylene blue dye had been considered suitable for the detection of microleakage due to having a molecular size similar to or smaller than that of bacterial products [42]. Protaper universal system was used in this study for reducing the time required for biomechanical preparation and improving the standardization of instrumentation [43].
Based on the statistically significant differences among the three canal filling materials for permanent teeth evaluated in this study, it was concluded that the overall sealers consisting of nanoparticles presented lower leakage of dye than the zinc oxide based sealer which presented the highest leakage among the tested materials. This was in agreement with Meidyawati et al., 2017 [44], who found that the MTA Fillapex sealers contain nanoparticles of MTA powders and rosins (resin), resulting in good flowability that allows penetration of the material into the main and lateral root canals and dentinal tubules.
Also with the study of Liang-Jiab BI et al., 2006 [45] which stated that the HA nanoparticles can effectively seal the exposed dentinal tubules.
A possible reason for this difference in the sealability between nanoparticles and traditional sealers was related to the size of the nanoparticles with a diameter of 100 nm or less which provided them with a greater contact surface area and charge density than bulky powders.
Penetration of sealer inside the tubules is potentially useful because it increases the interface between the core material and dentinal walls, which may improve the mechanical retention of the material via sealer plug interlocking inside the tubules [46]. It was proven that the addition of nanoparticles did not deteriorate the flow characteristics of the sealer and reduced viscosity, leading to enhanced flow of the sealer [47]. Also using nano-sized materials, the anti-leakage property of the sealer can be enhanced [48].
In the present study, ZnoE sealer allowed the highest leakage level and nano Ca(OH)2 sealer allowed the lowest leakage level of dye. This finding was found to be in agreement with Sleder et al., 1991 [49]. who found that Ca(OH)2 sealer had a sealing ability comparable to ZnoE sealer and can withstand long term exposure to tissue fluids without significant leakage.
The reason for the significant difference between the groups where Ca(OH)2 based sealers and zinc oxide eugenol based sealer were used could be that zinc oxide eugenol is water-soluble and has the disadvantage of being decomposed by water through a continuous loss of eugenol. This makes zinc oxide eugenol a weak unstable material. This is in agreement with Patel et al., 2007 [50] who proved that zinc oxide eugenol sealer in spite of its popularity as being a sealer with decades of clinical and laboratory application, is considered one of the sealers with the least penetration ability and highest leakage value. This was also in agreement with Balguerie et al., 2011 [51] who found little or no penetration of zinc oxide eugenol based sealer in the apical third of specimens.
Ca(OH)2 has been transported or mechanically forced into the dentinal tubules and thus occluded the dentinal tubules, blocking them off and decreasing dentinal permeability [52].
Nano bioactive glass sealability can be explained by the alkaline nature of bioceramic by-products reported to denature collagen fibers, which facilitates the penetration of sealers into the dentin tubules (Balguerie et al., 2011) [51].
CONCLUSION
The results of this study showed that synthesized nano-powder sealers exhibited less microleakage in comparison with ZOE, making them suitable for use in root canal treatment. Nevertheless, further studies should be carried out and limitations and the potential unknown risks involved in the use of nano-powders as a medical material should be considered to verify their safety.
AUTHORS’ CONTRIBUTIONS
Dr. Asmaa Ahmed Desouky had contributed to Literature search, Clinical studies, Manuscript preparation, Manuscript editing, worked as a Guarantor. Prof. Maged Mohamed Negm had contributed to study Concepts, Design, Experimental studies (Amount of dye penetration) and Manuscript review. Prof. Magdy Mohammed Ali worked on Definition of intellectual, Data analysis, and Statistical analysis. Finally, approval of the final version of the manuscript for publication was given by all authors.
ETHICAL APPROVAL CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data sets analyzed during the current study are available from the corresponding author on request.
FUNDING
None.
CONFLICTS OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Many thanks to Prof. Yasser FathiHussien, the former head of material department, Faculty of Dentistry, Minia University, for continuous support and valuable discussion.