Periosteal Envelope Flap as a Technique for Horizontal Bone Augmentation: A Case Series Study
Abstract
Background:
Following tooth extraction, the alveolar bone is typically subject to irrevocable and progressive changes that are collectively referred to as natural bone resorption. This process eventually results in a deficiency of the vertical and horizontal dimensions of the bone. Conventionally, various methods are used to repair alveolar defects resulting from tooth extraction, and to achieve vertical or horizontal bone regeneration. The aim of this study was to evaluate the influence of periosteal pocket flap on the enhancement of horizontal length in alveolar bone regeneration.
Methods:
Twenty-two patients (7 men, 15 women) aged 45–60 years were enrolled in this study. Periosteal envelope flaps and Cerabone were used to increase alveolar bone thickness. Ridge width was measured preoperatively and 4-6 months postoperatively using cone-beam computed tomography. The pre- and postoperative results were compared using the paired t-test.
Results:
An average of 2.53 mm (P < 0.001) horizontal enhancement of the alveolar ridge was achieved.
Conclusion:
The results of this study suggest that the use of a periosteal pocket flap with xenograft material is an excellent method which increase more than 2 mm alveolar bone width. As the study sample was small, further clinical investigations with larger samples are recommended.
1. INTRODUCTION
Implant therapy is known as the best method for the restoration of edentulous sites [1]. Prosthetic implantation requires the presence of sufficient high-quality bone. Resorption of the alveolar process may occur as a consequence of inflammation, trauma, or tooth extraction [2]. Following tooth loss, the adjacent bone resorbs to a greater extent horizontally than vertically in the anterior and posterior regions of the mouth [3, 4]. Some reports describe a 50% decrease in the horizontal dimension of the bone during the 12 months following tooth loss, with two-thirds of this loss occurring within 3 months after extraction [5]. At its onset, bone resorption is observed mainly on the buccal surface; it affects the lingual surface in its advanced stages. Undesirable intermaxillary relationships, periodontal disease, congenital tooth absence, developmental defects, tumors, dehiscence, and fenestration may contribute to bone resorption [6-8]. Clearly, sufficient bone volume is a prerequisite for the long-term success of an implant, as it permits correct three-dimensional placement and ensures stability [9]. Therefore, investigations are underway to identify more convenient and effective surgical methods to enhance alveolar bone length.
Steigmann et al., [10], introduced the periosteal pocket flap, which can be used with Bio-Oss® to provide adequate coverage, increasing the alveolar ridge length. The authors reported a nearly three-fold increase in bone length after 6 months of follow-up. Buser and Dula [11] achieved a 98.3% success rate in 61 implantation cases treated previously with autografts and non-absorbable barrier membranes. Fugazzotto [12] used Guided Bone Regeneration (GBR) and reported cumulative success rates of 97.2% in the mandible and 97.4% in the maxilla for 607 implants, the function of which was monitored for up to 133 months. In a systematic review, the survival rate of implants placed in regenerated bone resulting from GBR was similar to that of implants placed in native bone [13]. Another systematic review of the clinical use of grafting materials between 1995 and April 2015, which included 184 papers from the PubMed and Cochrane databases indicated that combined average horizontal and vertical gain of 3.7 mm can be achieved using granular materials [14]. Sanz-Sanchez et al., [15], conducted a systematic review and meta-analysis of 40 clinical studies to assess the efficacy of lateral bone augmentation in relation to alveolar crest dimensions; they found that the use of bone replacement grafts with barrier membranes and the application of autogenous bone blocks both led to high (>95%) success and survival rates.
GBR is a technique used for ridge augmentation. It involves the surgical placement of an absorbable or non-absorbable barrier membrane to stabilize the fibrin clot, maintain a space for osteogenic cells, and exclude cells that impede bone formation (e.g., epithelial cells and fibroblasts) [16-18]. One of the most prevalent problems associated with GBR is dehiscence of the soft tissue, which is detrimental to wound healing. To ensure the success of GBR, primary wound closure has been proven to be essential [19-22].
Bone autografts continue to be the gold-standard graft materials for bone augmentation due to their remarkable osteogenic properties, which enable harvesting from intraoral sources such as the maxillary tuberosity, chin, and mandibular ramus, as well as from extraoral sources such as the hip [23-26]. The most important risk to consider when using autografts is donor-site morbidity [27, 28].
When using GBR to achieve horizontal or vertical regeneration, the soft tissue should be mobile to attain effective closure. Soft-tissue dehiscence often necessitates premature removal of a non-absorbable barrier membrane, which is not true of a collagen membrane [29, 30]. Materials exposed to the intraoral environment degenerate more rapidly, jeopardizing the final outcome [22]. To avert exposure of the wound, the flap is usually applied and stretched to a great extent. The periosteum is then removed from its base. Making such an incision in dental periosteum is bound to damage the periosteal vessels and the upper vascular network, to the detriment of the local blood supply. The periosteal pocket flap was designed to address this issue. The periosteum is carefully removed from the mucosal coverage at the mucogingival junction with a blunt instrument, which is expected to maintain the integrity of the vessels in the periosteum-free mucosal flap and those within the periosteum, facilitating local blood supply. The mucosa is free and can passively and effectively cover the area; the membrane is placed under the periosteum and the periosteum is sutured to the lingual flap, which fixes the membrane completely, making the application of a fixture device unnecessary [10].
The aim of this study was to evaluate the effect of periosteal pocket flap use on the enhancement of horizontal length in alveolar bone regeneration.
2. MATERIALS AND METHODS
2.1. Subjects and Sample Collection
In this case-series study, samples were collected in a randomized and task-oriented order. Patients who were referred to professional periodontology clinics in the city of Tehran were enrolled.
The Board of Research and Ethics Committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran, approved this study and written informed consent was obtained from all participants. Patients admitted to a private clinic for implant-based therapy who had narrow ridges that did not permit conventional implant placement (e.g., synchronous implantation and augmentation) were eligible for this study. The treatment plan, including possible side effects and benefits of the procedure, was explained to the subjects. Patients were assured for the anonymity of their data. Comprehensive periodontal examination of the remaining teeth, including the assessment of pocket depth, bleeding on probing, and tooth mobility, was performed. Patients who smoked and those with systemic diseases (e.g., diabetes, osteoporosis), parafunctional habits, histories of immunosuppressive drug or bisphosphonate use, and/or edentulous areas encompassing more than four tooth sites were excluded.
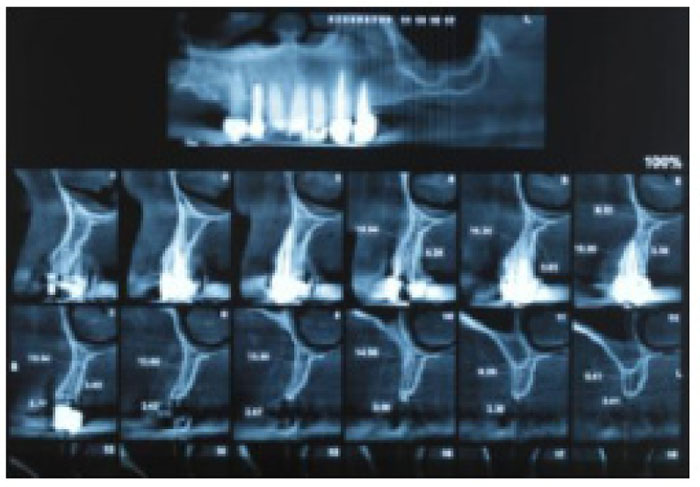
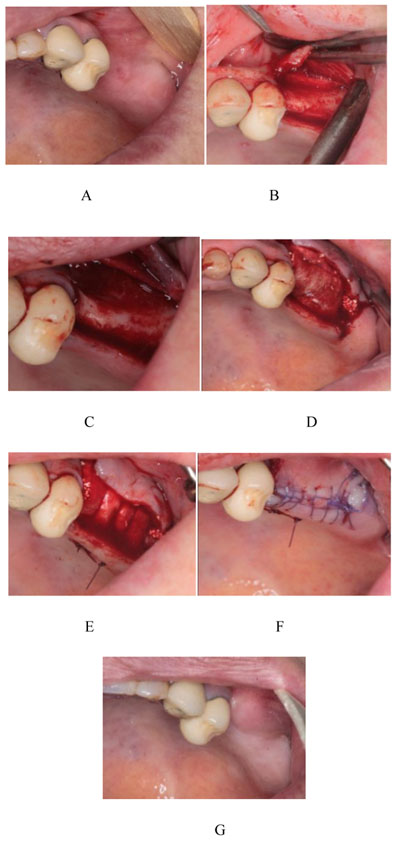
Prior to the procedure, patients were evaluated using Cone-Beam Computed Tomography (CBCT; ProMax 3D Mid; Planmeca Oy, Finland, Helsinki). On the images, ridge thickness was summed in 2-mm increments; the total was then divided by the number of sections to obtain an average ridge thickness for each patient. Patients with ridge widths < 4 mm were included in the study (Fig. 1). First, the patients were draped and prepared, including the perioral application of povidone iodine. Then, local anesthesia was achieved by administering one or two cartridges of 2% lidocaine with 1:80,000 epinephrine (Persocaine E; Darupakhsh, Tehran, Iran) at the injection site (Fig. 2A). Surgery was initiated 10 min later. Initially, a crestal incision was made to the desired length with a #15 scalpel. Then, a transverse line was delicately incised on the periosteum using a scalpel to create a shallow gap. The gap was then deepened with a periosteal elevator, completely detaching the mucosa from the periosteum (Fig. 2B). When necessary, a vertical releasing incision was made in the mesial area of the attached gingiva, proximal to the crestal incision and extending to the mucogingival junction. A similar incision was made in the distal area. At this stage, the mucosal flaps were lifted to the mucogingival junction using an elevator. Next, the periosteum was removed from the bone with a periosteal elevator to create a pocket equal in size to the intended augmentation (Fig. 2C). As no releasing incision had been made on the periosteum, this pocket was formed to accommodate the graft material and membrane. An absorbable collagen membrane (Jason) (Botiss Dental, Berlin, Germany) and a sufficient quantity of Cerabone powder (Botiss Dental, Berlin, Germany) were placed inside the pocket (Fig. 2D).
The membrane was positioned on the graft material so that its edge rested under the lingual flap. Then, the periosteum was sutured to the membrane, extending to the lingual flap, with a range of sutures (vicryl 0-3; SUPA Medical Devices, Tehran, Iran) (Fig. 2E). The buccal flap, which contained the alveolar mucosa and attached gingiva, was sutured to the lingual side (Fig. 2F).
2.2. Postoperative Instructions
After surgery, patients were prescribed amoxicillin (500 mg every 8 h; Kosar, Tehran, Iran) and analgesics (e.g., acetaminophen, codeine). Patients were advised to refrain from having any prosthesis placed in the area of the graft until 4 months postoperatively, and to consume a soft diet for 24 h following the operation up to 10 days. And then sutures were removed 2 weeks after surgery.
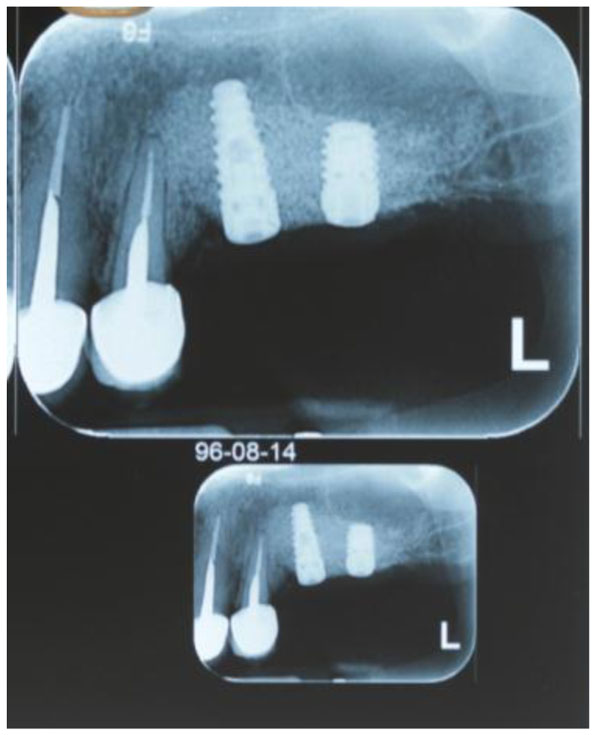
At 4 - 6 months postoperatively (Fig. 2G), follow-up CBCT images were obtained to determine the dimensional changes that had occurred and the appropriate implant size (Fig. 3). Preoperative and postoperative images were used to quantify the longitudinal enhancement of the ridge for each individual. Suitable implants were inserted subsequently (Fig. 4).
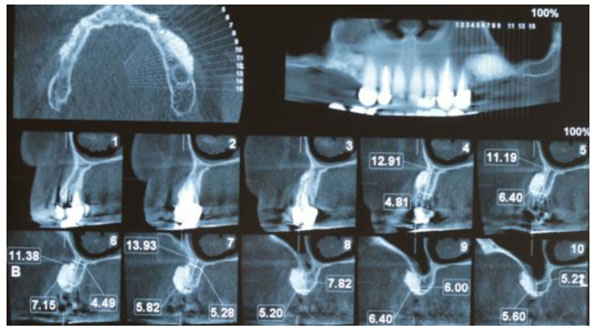
3. RESULTS
In total, 22 patients (7 (32%) men, 15 (68%) women) were enrolled in this study. The average age was 48.74 ± 8.79 (range, 40 - 65) years. The mean preoperative and postoperative ridge widths were 2.94 and 5.47 mm, respectively. The mean increase in ridge width (2.53 mm) was significant (P < 0.001, t = 13.21) (Table 1) no wound dehiscence or infection was recorded 4 - 6 months postoperatively.
| - | Mean | Min | Max | SD* | T-test Result |
|---|---|---|---|---|---|
| Pre op width | 2.94 mm | 1.98 mm | 4.3 mm | 0.57 | T=13.21 P< 0.001 |
| Post op width | 5.47 mm | 4.21 mm | 6.86 mm | 0.71 | |
| Differentiation | 2.53 | 2.23 | 2.56 | - |
The 22 patients had in total 24 implants installed and all of the implants were Biohorizons (Birmingham, USA) with standard diameter.
4. DISCUSSION
Endosseous implants have recently become a reliable treatment option for partially or completely edentulous patients. However, implant therapy requires a sufficient volume of high-quality bone to ensure the attainment of favorable aesthetic and functional outcomes. In some cases, implant placement is difficult or impossible due to bone resorption. Bone transplantation can be performed with various kinds of graft; the use of autogenous grafts for this purpose is currently considered to be the gold standard.
Autografts are osteogenic, osteoconductive, and osteoinductive [31]. They can be harvested from intraoral and extra oral sources. Although their use is highly advantageous, they pose minimal risks of infection and immunological reactions after grafting. Arab et al., [32] applied bone autografts harvested noninvasively with a trephine drill and achieved an acceptable increase in ridge width. However, the most important drawback of this type of graft is that it entails two simultaneous surgeries.
In this study, a xenograft material obtained from the mineral phase of bovine bone (Cerabone; Botiss Dental) was used. Cerabone® has a strong resemblance to human bone in terms of surface porosity and chemical composition. Xenografts are osteoconductive and osteoinductive [29, 33, 34].
The significant increase in mean bone width observed in this study was comparable to the results of studies which have been performed with more difficult techniques.
Buser and colleagues evaluated augmented ridge with autogenous bone. the mean ridge width increased from 3.7 mm to 7.1 mm and reported high survival rate for implant inserted in augmented bone. their result is more than of our paper that showed the autogenous bone is the gold standard for ridge augmentation yet. autogenous block graft and longer follow up period are the reasons for this impressive bone gain [20].
Funaki et al., used distraction osteogenesis techniques to increase ridge width by 2.7 that somewhat resembles to our study [35].
in the study by Kelly the tunnel technique with xenograft particle used for ridge augmentation, and up to 6 mm increasing in ridge width achieved. Their results were along with our study and showed that these techniques are predictable [36].
Moeintaghavi et al., in a study investigated demineralized cancelous bone bolck graft for horizontal ridge augmentation and showed 52/1 ± 28/1 mm enhancing in ridge width that is lower than that of our result. the limited number of patients and using cancelous block graft are the main causative factors of such weak bone gain [37].
Steigmann et al., used the periosteal pocket flap technique in atrophic ridge of 5 patients with xenograft and after 6 months cone beam computed tomography showed 389% increase in ridge width that is in line of our study but with significant more bone gain. The limited number of cases and limited area of edentulous ridge may be the reason of greater result of Steigmann study [10].
Periosteal pocket flap in this study provides predictable GBR outcome based on primary wound closure, wound stability along with angiogenesis. Primary closure provide the best environment for healing period without bacterial and mechanical disturbance that enhances surgical outcomes.
Angiogenesis, in this technique enhances the cortical perforation and wound stability. Decortication results in traveling pluripotent cell to migrate from bone marrow to the bone graft and to maximize mechanical interlocking between the graft and the host tissue.
This technique increases the stability of membrane and graft material with periost and periosteal suturing, even in cases of severe bone deficiencies.
Clot stabilization is the key factor for optimal healing outcome with recruiting cell and growth factors in the wound site and this results in predictable regeneration [19, 20, 22, 29, 32].
In this article, a 35% soft tissue dehiscence has been shown after GBR procedure. Without primary wound closure, significant reduction in bone regeneration has been observed and bacterial colonization in healing period has been the reason for this phenomenon which jeopardized the GBR results [22].
Friedman et al., [21], used cross-linked collagen membranes to achieve alveolar crest augmentation in 16 patients, and observed wound disruption and exposure in 62.5% (10/16) of cases. Norton et al., [38] used bovine and collagen membranes for sinus augmentation and employed GBR to increase horizontal thickness. The exposed membranes were observed in 26% of cases, two of which showed little or no new bone formation on histological examination. Hiatt and Schallhorn [39] showed that proper soft-tissue coverage increased the likelihood of bone regeneration. Three types of complication were observed in a study involving vertical augmentation with demineralized bovine bone and implant placement; one of these was the complete loss of augmentation after soft-tissue defects in 3 - 8 weeks earlier [40]. Thus, membrane exposure is a common and disruptive complication of GBR. Because the periosteal pocket technique permits tension free closure and hypermobility of the mucosa flap the number of soft tissue dehiscences decreased.
The strengths of this study include our demonstration that the periosteal pocket flap enables prevention of postoperative wound dehiscence and the achievement of full membrane coverage with soft tissue without flap stretching. Nonetheless, the study has several limitations, including the small sample. In addition, no histological examination or long-term follow-up was performed after the implantation procedure.
CONCLUSION
This study demonstrated that the use of a periosteal pocket flap with GBR provided an acceptable increase in ridge width. The authors recommend further clinical studies with larger samples, postoperative histological examination, and long-term follow-up.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The Board of Research and Ethics Committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran, approved this study.
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Written informed consent was obtained from all participants before their participation.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


