All published articles of this journal are available on ScienceDirect.
Biological and Immunogenicity Property of IgY Anti S. mutans ComD
Abstract
Objective:
This study aims to elucidate the effect of IgY anti ComD on the biological properties of Streptococcus mutans. (S. mutans) ComD is an interspecies quorum-sensing signaling receptor that plays an important role in biofilm formation by S. mutans.
Materials and Methodology:
Egg yolk IgY was produced by the immunization of chickens with a DNA vaccine containing the ComD DNA coding region. We evaluated the effect of the antibody on biofilm formation by S. mutans isolated from subjects with or without dental caries. We also assessed the immunoreactivity of the antibody against all isolates, and analyzed the protein profile of S. mutans by SDS-PAGE.
Results:
The ComD antibody was successfully induced in the hens’ eggs. It inhibited biofilm formation by all S. mutans isolates. In addition, the expression of some protein bands was affected after exposure to the antibody.
Conclusion:
IgY anti-S. mutans ComD reduces biofilm formation by this bacterium and alters the protein profile of S. mutans.
INTRODUCTION
The effectiveness of active immunization for the prevention of caries has been reported for animal models, but active immunization is not applicable in humans due to side effects. A passive immunization approach may therefore represent an alternative option for the prevention of dental caries caused by bacteria. As in other bacteria, the expression of the virulence factors of dental plaque-forming bacteria involves interspecies communication mechanisms, or quorum sensing [1-4]. Interventions affecting this communication system can be used as a strategy to control the colonization of the bacteria that cause caries. In S. mutans, communication involves the role of autoinducer molecules and their receptors (ComD) at the cell surface [3, 5-8]. DNA vaccine technology is more practical than protein vaccines [9-11] and it may be used for dental caries prevention, for example to induce the formation of specific IgY that inhibits the formation of dental plaque. The inactivation of genes encoding ComD decreases the potential of S. mutans to form colonies and to interact with other bacteria commonly found on tooth surfaces, such as Actinomyces viscosus and Lactobacillus spp. [6-8]. A study by Li et al. revealed that the inactivation of ComD and ComE in S. mutans resulted in an acid-sensitive phenotype and reduced biofilm formation [3]. Furthermore, the ComD mutant had a decreased ability to adhere to a mucin-coated polystyrene surface. Immunization of chickens with vaccines containing ComD genes in an expression vector with a eukaryotic promoter may improve the immunogenicity of vaccine [12-14] and results in the production of a specific IgY antibody against the ComD protein. Based on these findings, we aimed to test the ability of a specific IgY against the ComD receptor molecules to block the biofilm formation by S. mutans and to evaluate whether IgY anti ComD alters the S. mutans protein profiles.
One factor that affects the virulence of S. mutans is genetic expression, which is described through the protein profile. The protein profile reflects virulence factors that might be affected by changes in the microenvironment. Based on several studies, the most commonly studied proteins, which are assumed to have the most influence on the virulence of S. mutans, are antigen I/II, glucosyltransferase (Gtf), and glucan-binding protein (Gbp) [15, 16].
MATERIALS AND METHODOLOGY
Production of DNA Vaccine and Immunization of Chickens
The DNA vaccine we used contained pCDNA-ComD, which we had constructed previously [17]. This study was approved by the ethics committee of the Universitas Indonesia Faculty of Dentistry. Ten adult hens of large breeds were injected intramuscularly with 50 μg of the DNA vaccine isolated from E. coli (Dh5α). This immunization was carried out once in each of week 1, week 3, and week 6, after which the titer of antibodies in the blood was measured, and the eggs were collected and stored until they were used for IgY purification. The IgY from the egg yolk was purified using the EGGstract purification system (Promega, Madison, Wisconsin USA). Egg yolks were separated with an egg separator for further separation of the fat, which resulted in the water-soluble fraction of IgY. Egg IgY deposition was conducted in accordance with the manufacturer's recommendations. Quantification of IgY specific to S. mutans ComD was determined using the Enzyme-linked immunosorbent assay (ELISA) technique. The concentration of the water-soluble fraction of IgY, which was used in further experiments, was 0.14%.
Bacterial Culture and Biofilm Assay
S. mutans cells were collected from subjects with and without dental caries. Bacteria were cultured in Trypticase Soy Broth Agar (TYSBA) for 48 h prior to being used for biofilm assays, immunogenicity testing, and protein band profiling.
The effect of IgY anti S. mutans ComD on S. mutans biofilm formation was evaluated. A crystal violet assay was performed to determine the quantity of biofilm formed [18]. Briefly, isolates were grown overnight in TYSB broth at 37°C. Cultures were diluted at 1:20 in fresh LB broth at room temperature, and 200 uL of this suspension was used to inoculate sterile, 96-well polystyrene microtiter plates (Iwaki, Tokyo, Japan). After 24 h at 37°C, the biofilms grown on microtiter plates were washed with PBS, dried in an inverted position, and stained with 1% crystal violet for 15 min. After rinsing with deionized water, the crystal violet was solubilized in 200 uL of ethanol acetone (80:20, vol/vol), and quantitated by adding 95% ethanol followed by measurement of the absorbance at 595 nm.
Immunogenic Properties of IgY
To determine the immunogenic properties of IgY, ELISA was performed by incubating S. mutans cultures with appropriate test groups of IgY and control solutions. S. mutans culture isolated from caries and free caries subject were coated in microplate plate prior incubated with IgY overnight at 40°C. The next day the plate were washed with phosphate buffer saline Tween 20 (PBST) (Sigma, St. Louis, MO USA) followed by incubation with Goat Anti-Chicken IgY H & L (HRP) (abcam, Cambridge, UK) for 1 hour at 37°C. After washed with PBST, 3,3’,5,5’-Tetramethylbenzidine (TMB) (Bio-Rad, Bio-Rad, Platelia, USA) substrate was added and HCl 0.1 N was used to stop the enzymatic reaction. The optical density of each group was determined using a microplate reader at 450 nm [12].
S. Mutans Protein Expression Analysis
We evaluated the effect of IgY anti ComD on the protein expression of S. mutans. We collected the cells by centrifugation (13.000 rpm, 1 min). Each of the collected pellets was washed once with PBS and homogenized using an ultrasonic homogenizer (Omni-Ruptor 250, OMNI International Inc.) The protein expression of S. mutans was analysed by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE).
RESULTS
We found that IgY anti ComD showed immunoreactivity properties against both isolates of S. mutans (from subjects with and without dental caries; (Fig. 1). The immunogenicity of the IgY was greater against S. mutans from subjects with caries than for cultures from caries-free subjects, but the difference was not statistically significant (P > 0.05). The capacity of both isolates to form a biofilm was suppressed by the antibody (Fig. 2).
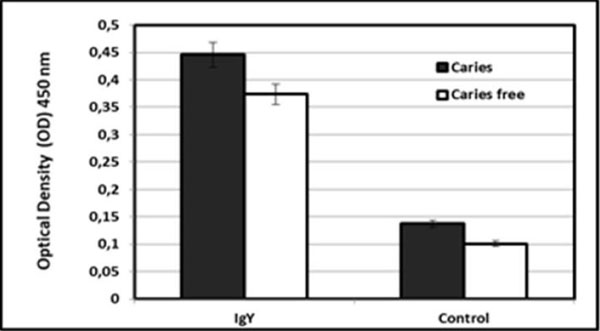
IgY anti S. mutans ComD immunogenicity against S. mutans isolated from caries and caries free subjects.
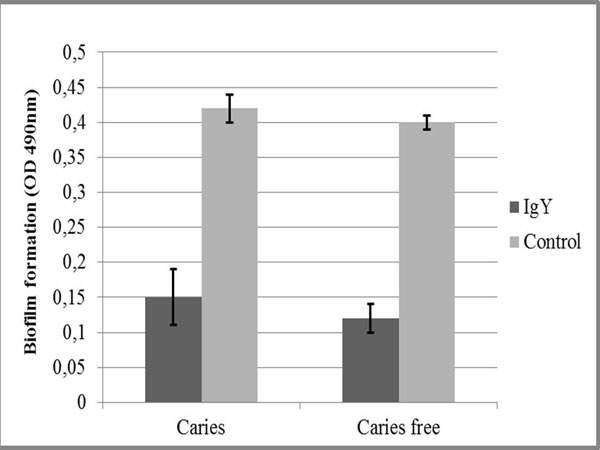
Biofilm formation of S. mutans on caries and caries free subject after exposure IgY anti S. mutans ComD.
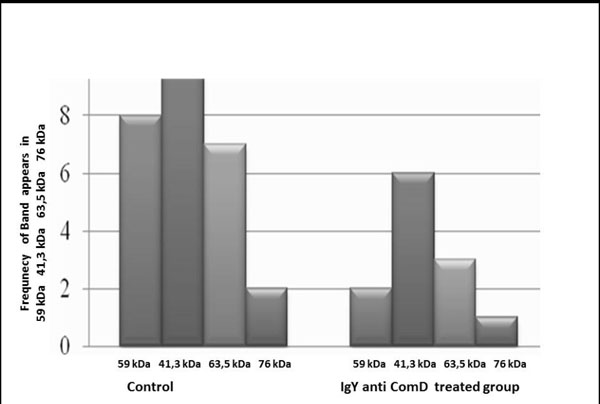
Effect of IgY anti S. mutans ComD in the protein expression of S. mutans from caries free subjects.
The SDS-PAGE analysis showed that the expression of some protein bands (41.3 kDa, 59 kDa, 63.5 kDa, and 76 kDa) was downregulated by the antibody in the isolates from caries-free subjects. Proteins with a molecular weight of 59 kDa were expressed in eight of the isolates from the control group, and only in two from the treated group. In the isolates from subjects with caries, the expression of proteins with a molecular weight of 41.3 kDa, 63.5 kDa, or 76 kDa decreased in the treated group relative to the control group (9, 7, and 2 versus 6, 3, and 1; Fig. 3).
The antibody affected protein expression in isolates from subjects with caries, decreasing protein expression to similar levels as in isolates from caries-free subjects, except for a 76 kDa protein. The expression of proteins with molecular weights of 41.3 kDa, 59 kDa, and 63.5 kDa decreased in the treated group relative to the control group (8, 8, and 7 versus 5, 5, and 3; Fig. 4). (Fig. 5) shows a representative protein profile for this study.
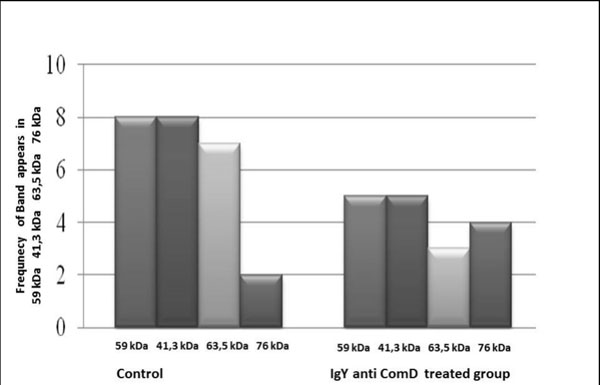
Effect of IgY anti S. mutans ComD in the protein expression of S. mutans from caries subjects.
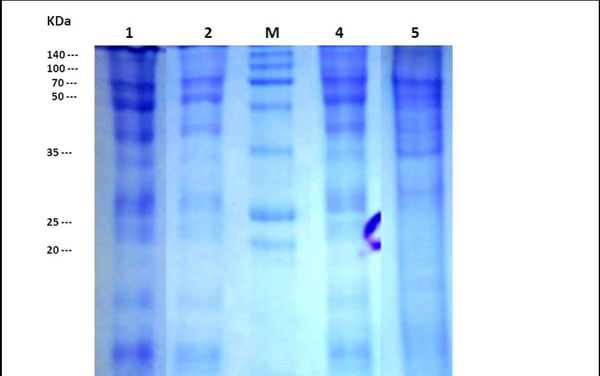
Profile of protein bands of S. mutans. Lane 1: S. mutans isolated from caries-free subject (control), Lane 2: S. mutans isolated from caries subject after incubation with IgY anti S. mutans ComD, M : Marker, Lane 4: protein band from S. mutans isolated from caries-free subject after incubation with IgY anti S.mutans, Lane 5: S. mutans isolated from Caries subject (control).
DISCUSSION
The ComC mutant, unable to produce or secrete competence-stimulating peptide (CSP), forms biofilms with an altered architecture, whereas the ComD and ComE mutants, which are defective in sensing and responding to CSP, form biofilms with reduced biomass [3, 18, 19]. The results of this study indicate that IgY anti ComD can inhibit biofilm formation by S. mutans cells isolated from subjects with or without caries. This suggests a promising strategy for providing a level of protection from dental caries caused by these organisms.
This finding is similar to the effectiveness of active immunization with Glucan Binding Protein B(GBP-B), via systemic [20] or mucosal [21] routes, in preventing the adhesion of S. mutans onto tooth surfaces. The present study, however, is the first to demonstrate that an antibody to ComD from S. mutans can be protective when passively administrated. This phenomenon could be because the quorum sensing system in S. mutans also affects acidogenicity, aciduricity, genetic transformation, and bacteriocin production [1]. Furthermore, the protective effect may apply to subjects with all levels of susceptibility or resistance to dental caries.
Our study suggests that using the water-soluble fraction of IgY yields good results, which might be a valuable finding to support further research into clinical applications in the form of mouth rinse, toothpaste, or other oral care products. Our results showed that a solution of 0.14% IgY antibody can inhibit the biofilm formation of S. mutans in vitro. They also demonstrated the immunoreactivity of IgY anti S. mutans ComD against S. mutans isolated from subjects with and without dental caries. In addition, application of this antibody resulted in the suppression of proteins that may be glucan-binding [22-24]. The protein profile reflects virulence factors that might be affected by changes in the microenvironment; for example, Decker et al. reported that the gene expression of ComD in a xylitol-supplemented medium was significantly higher than that in a sucrose-supplemented medium [25].
CONCLUSION
IgY anti-ComD can decrease biofilm formation and affect the bacterial protein expression of S. mutans.
CONFLICT OF INTEREST
None declared.
ACKNOWLEDGEMENTS
This study was supported by the Grant from The Universitas Indonesia 2011 and Competence Grant from Indonesian Ministry of Education 2013. Authors were grateful for the assistance of Dessy and Maysyaroh from Oral Biology Laboratory Faculty of Dentistry University of Indonesia and Okti from Immunology Department of Veterinary Faculty, IPB. Bogor.


