All published articles of this journal are available on ScienceDirect.
Root Surface Bio-modification with Erbium Lasers- A Myth or a Reality??
Abstract
The objective of this literature review was to critically review the evidence available in the literature regarding the expediency of erbium family of lasers for root bio modification as a part of periodontal therapy. The literature search was performed on the Pubmed using MeSH words such as "lasers/therapeutic use, scaling, dental calculus, tooth root/anatomy and histology, ultrasonic therapy". The studies were screened and were grouped as follows: those evaluating a) efficacy for calculus removal with the Erbium family of laser b) root surface changes following Er YAG and Er Cr YSGG application c) comparative studies of the Er YAG, Er Cr YSGG lasers versus conventional methods of root surface modification d) Bio compatibility of root surface following Erbium laser treatment e) Studies on the combined efficacy of laser root modification with conventional methods towards root surface bio-modification f) Studies on effectiveness of root surface bio-modification prior to root coverage procedures. In conclusion, the erbium family has a proven anti-bacterial action, predictable calculus removal, minimal root substance removal, and appears to favor cell attachment. The Erbium family of lasers appears to be a useful adjunct for the management of periodontal disease.
INTRODUCTION
One of the most challenging aspects of periodontal therapy is the development of a predictable approach for root surface modification. Root surface modification gains importance due to the fact that the biofilm contributes to root surface changes which impair regeneration attempts. In order to achieve an understanding of why regeneration attempts succeed or fail, knowledge of root surface changes in periodontal disease and its clinical implication becomes essential.
A BRIEF OVERVIEW OF ROOT SURFACE CHANGES IN PERIODONTAL DISEASE
The etiology of periodontitis is bacteria which attach to the root surface and thrive in a biofilm environment. These bacteria later become partially mineralized to form calculus. A portion of the bacteria remains unattached, float freely in the gingival crevicular area and are commonly responsible for tissue invasion of the periodontium, resulting in a variety of host bacterial interactions which manifest clinically as periodontal disease [1].
The root surface undergoes a series of changes in its physical, chemical nature and also becomes cytotoxic due to the release of bacterial toxins that get attached to the root cementum. Broadly, the changes include a loss of fiber attachment from the cementum area (physical) [2], demineralization of the root surface forming craters/ root caries (chemical changes) [3] and lipopolysaccharide attachment to cementum surface (necrotic / altered cementum)[4].
Rationale for Root Bio-modification
The factors influencing successful periodontal therapeutic outcomes (regeneration) include [5]
- Clot stability.
- Cell migration towards the root surface.
- Cell attachment.
- Cell proliferation and differentiation.
The root surface properties play an important role in all the above mentioned events as established by Polson and Caton [6]who postulated that ideal root surface should be free of contaminants and superficial layer of hypermineralized cementum. Therefore preparation of a root surface which is favorable for the above mentioned events constitutes the rationale behind root bio-modification.
Root Modification/Bio-modification
Root bio-modification refers to procedures which are done to de-toxify, de-contaminate and de- mineralize the root surface, thereby removing the smear layer and exposing the collagenous matrix of dentin and cementum [7]. The oldest and the most conventional methods include scaling and root planing which are primarily aimed at the gross removal of microbes from the root surface. The scaling and root planing had a limitation in that; it also resulted in removal of cementum and formation of smear layer [8]. This was followed by the advent of root conditioning agents,
Root conditioning agents aimed at the removal of the smear layer [9] produced during scaling and root planing to expose the dentinal tubules and dentinal collagen (to favor the joining of the Sharpey’s fibers with root collagen). This modality gave way to application of fibronectin and recombinant forms of growth factors such as PDGF, BMP, enamel matrix proteins [10]. These factors provided the signals for the cell chemotaxis, attachment, proliferation and differentiation. The use of recombinant growth factors for root bio-modification is limited as they are expensive and cannot be used as a mono-therapy. In addition, a cocktail of growth factors which act in a sequential manner are needed to obtain a successful periodontal regeneration. Amongst, the above mentioned modalities of treatment only EMDOGAIN (enamel matrix proteins) have demonstrated new cementum formation [11].
A significant step towards successful periodontal regeneration could be attained if the surface contaminants could be removed with minimum damage to underlying cementum. In this regard, the Erbium lasers represent a potential therapeutic tool. A summary of the various root bio-modification agents used are provided in Table 1.
Root conditioning agents used in periodontal therapy.
| Agent used | Method of use | Advantages | Limitations |
|---|---|---|---|
| Citric Acid | pH 1.0. Topical application on root surface for 2-3 minutes after scaling and root planing. | Removal of smear layer [9]. | No predictable regeneration [12]. pH acidic and can be unfavorable for cell attachment [13]. |
| Tetracycline | 100mg/ml solution. Topical application on root surface for 5 minutes after scaling and root planing. | Anti-collagenase and anti-microbial activity [14]. Smear layer removal [15]. |
No significant gain in attachment reported [12]. |
| Fibronectin | 0.38mg/ml solution- topical application. | Promotes cell adhesion to root surface and chemotactic effect on periodontal fibroblasts [16]. | Ineffective when used alone. When combined with citric acid, better clinical attachment level gain [17]. |
| EDTA | 2 concentrations have been used (8% and 24%). The 24% concentration had neutral ph (7.0-7.2). Topical application on root surface for 2-3 minutes after scaling and root planing | Effective smear layer removal [18]. Neutral pH favors cell migration and attachment [19]. |
Ineffective when used alone. When used with Emdogain, periodontal regeneration has been demonstrated [20]. |
| EMDOGAIN | A combination of enamel matrix proteins. Bio-mimetic concept. Used along with 24% EDTA. |
Acellular cementum formation has been demonstrated [21]. | Cost is prohibitive |
Summary of various studies performed comparing the efficacy of calculus removal using Erbium laser and conventional methods.
| Author and Year | Study groups and Methodology | Findings and Conclusion |
|---|---|---|
| Aoki A et al.
2000 [27] |
53 periodontally compromised teeth Ultrasonic scaling Vs Erbium Laser (40 mJ per pulse and 10 pulses under water spray) |
Laser scaling provided a level of calculus removal that was similar to that provided by the ultrasonic scaling. The Er:YAG laser produced superficial, structural and thermal microchanges on the root cementum. |
| Schwarz F et al. 2001 [28] | Forty single rooted teeth Ultrasonic scaling Vs Erbium Laser (120 mJ, 140 mJ, 160 mJ, 180 mJ at 10 Hz) |
Er:YAG laser resulted in a smooth root surface morphology, even at higher energy settings. The results also seem to indicate that calculus removal can be selectively done in vivo |
| Frentzen M et al. 2002 [29] | 40 extracted teeth Ultrasonic scaling Vs Er YAG Laser (160mJ/pulse, 10 Hz.) |
Laser scaling was accompanied by an increased removal of tissue and roughened surfaces. Laser scaling resulted in an increased loss of cementum and dentin. |
| Eberhard J et al. 2003 [30] | The mesial and distal surfaces of 30 single-rooted teeth with untreated periodontitis were treated either by hand instrumentation (scaling and root planing (SRP)) or by Er:YAG laser irradiation (160 mJ, 10 to 15 Hz) | Following laser irradiation, 68.4±14.4% of the root surface was calculus free in contrast to 93.9±3.7% after SRP when both treatments were performed for the same time (2:15±1:00 min). The histologic evaluation showed that after SRP 73.2% of root dentin was completely denuded from cementum, while only a minimal cementum reduction was apparent after laser irradiation. |
| Schwarz F, et al. 2006 [31] | 72 single-rooted teeth (n=12 patients) were randomly treated in vivo by a single course of subgingival instrumentation using (1-3) an Er: YAG laser (ERL1: 100 mJ; ERL2: 120 mJ; ERL3: 140 mJ; 10 Hz), or (4) the Vector ultrasonic system (VUS) or (5) hand instruments (SRP). | Highest values of Residual subgingival calculus areas (RSC) (%) were observed in the SRP group (12.5±6.9). ERL (1-3) (7.8±5.8, 8.6±4.5, 6.2±3.9, respectively) revealed significantly lower RSC areas than SRP. VUS (2.4±1.8) exhibited significantly lower RSC areas than SRP and ERL (1, 2). |
| Moghare Abed A et al. 2007 [32] |
The mesial and distal surfaces of 15 periodontally loose extracted teeth were treated randomly either by hand instrumentation or by Er:YAG laser irradiation. (160 mJ, 12 Hz) | The surface roughness in Er: YAG laser group was more than in hand instruments group. Lower frequency and long pulse duration maybe more suitable for the micro-morphology of root surface after treatment. |
| Krause F et al. 2007 [33] | 20 teeth were treated with an Er: YAG laser. Laser settings were 140 mJ and 10 Hz. | The amount of residual calculus following laser irradiation depends on the fluorescence threshold level for a feedback-controlled Er: YAG laser. |
| Ting CC et al. 2007 [34] | 65 non carious teeth were prepared and divided randomly into three groups: a control group (N=8), irradiation without water group (no water [NW] group; N=39), and an irradiation in water group to simulate the conditions in a periodontal pocket group (in water [IW] group; N=44). The power output settings for Erbium laser irradiation were 0.5, 1.0, 1.5, and 2.0 W for each group. | Mean Ra and Z values in the IW group were significantly higher than in the NW group with the same power output. Thermal alterations were completely absent in the IW group. With regard to efficiency of calculus removal, the 0.5 W setting (0.11±0.036 mm2/second) was significantly inferior to the 1.0-W setting (0.27±0.043 mm2/second). The 2.0-W setting (0.63±0.272 mm2/second) was much more efficient but resulted in significant morphologic alterations. |
| Hakki SS et al. 2010 [35] |
32 single-rooted teeth were treated by different methods including (1) conventional hand instruments; (2) hand instruments and tetracycline-hydrochloride (Tet-HCl); (3) erbium, chromium:yttrium-scandium-gallium-garnet (Er,Cr:YSGG) laser irradiation, setting I (short pulse); (4) Er,Cr:YSGG laser irradiation, setting II (long pulse). | Roughness was greater in the long-pulse laser setting than in the short-pulse setting. All treatments were equally efficient in calculus removal from the root surfaces. |
| Oliveira GJ et al. 2012 [36] | 60 teeth samples were divided into 3 groups (20 each) Group 1: Control (G1). Group 2: Er Cr YSGG laser irradiation (G2). Group 3: Er YAG laser irradiation (G3). Out of the 20 samples in each group, 10 samples received blood application. Root surface changes and blood component adhesion was assessed by Scanning Electron Microscope (SEM). |
Teeth treated with Er: YAG and Er Cr YSGG lasers demonstrated greater root surface roughness than those in the control group. Er YAG laser treatment allowed a greater degree of blood component attachment as compared to exposure to Er Cr YSGG laser. |
| Alhmedi A et al. 2013 [37] | A comparison of cementum alterations following treatment with Er YAG and CO2 laser using non de-calcified thin histologic sections was done. Parameters were as follows: 1. Er YAG laser was used with the following parameters 40 mg/ pulse (14.2J/cm2/ pulse) and 25 HZ (1.0W) under water spray. 2. Co2 laser irradiation was performed in non contact mode at 1.0 W 3. Ultrasonic scaling was used a control group. |
Er YAG treatment group demonstrated micro-irregularities with whitish slightly ablated surface and thermal changes up to 20 microns. In Co2 laser group a carbonization and thermal changes up to 140 microns thickness was observed. The authors concluded that Er YAG laser with water cooling resulted in minimal thermal damage. |
Studies summarizing the biological reaction of the cells to erbium treated root surfaces.
| Author and Year | Study protocol | Findings and Conclusion |
|---|---|---|
| Schoop U et al. 2002 [46] | Assessed the impact of Er YAG laser on root surface morphology and its ability to facilitate adhesion of mouse fibroblasts | Er YAG laser irradiated root surface offers better condition for adherence of mouse fibroblasts |
| Schwarz F et al. 2003 [47] | In vivo effects of Er:YAG laser (100mJ, 10 Hz) on the biocompatibility of periodontally diseased root surfaces in cultures of human periodontal ligament fibroblasts (PDL). | Erbium lasers promote attachment of PDL fibroblasts on previously diseased root surfaces. The surface structure of Erbium laser instrumented roots offer better conditions for the adherence of PDL fibroblasts than scaling and root planing. |
| Feist IS et al. 2003 [48] | Adhesion and growth of cultured human gingival fibroblasts. | Surfaces treated with 60 mJ/pulse Er:YAG laser irradiation promoted faster adhesion and growth as compared to surfaces treated with either root planing or 100 mJ/pulse Er:YAG laser irradiation. |
| Crespi R et al. 2006 [49] | Laser (160 mJ/ 10 Hz) vs Ultrasonic scaler in the treatment of periodontally diseased teeth. The teeth were incubated in fibroblast suspension and cell attachment and density was assessed. | Laser-treated specimens showed a significantly higher cell density number compared to untreated control surfaces and ultrasonically treated surfaces. |
| Theodoro LH et al. 2006 [50] |
The authors assessed the stability of the fibrin clot on the root surfaces irradiated with Er YAG and diode laser (808nm) using the scanning electron microscopic technique. 5 groups of 20 tooth samples each were taken and were divided as follows: G1 (no treatment), G2 (Er YAG-7.6 J/cm2), G3 (Er YAG- 12.9 J/cm2 ), G4 (Diode laser- 90J/cm2), G5 (Diode laser- 108 J/cm2). |
The authors demonstrated comparable fibrin clot formation and adhesion in root planing group and Er YAG group. In the diode group, a scarce network of fibrin and absence of cells was observed indicating the poor fibrin clot response to the diode group. |
| Galli C et al. 2009 [51] | Cell morphology using periodontal ligament fibroblast culture investigated by SEM after 3, 6, 24, and 48 hours of culture. | The surface changes produced a less favorable environment for cell adhesion or growth, and treated dentin seemed to be more suitable for periodontal ligament fibroblasts adhesion as compared to human osteoblast adhesion. |
| Hakki SS et al. 2010 [52] | Attachment of Periodontal ligament fibroblasts to periodontally involved root surfaces treated with Erbium laser with short pulse and long pulse setting. | Short-pulse laser setting may enhance the attachment, spreading, and orientation of Periodontal ligament cells |
| Galli C et al. 2011 [53] | Cell viability and production of osteocalcin and osteoprotegrin by osteoblast cell line plated onto titanium surface modified discs following irradiation of the discs with Er YAG laser at two different settings: 150 and 200 mJ/pulse at 10 Hz. | Erbium lasers produce changes on the surface of titanium disc that can negatively affect the viability and the activity of osteoblast. |
| de Oliveira GJ et al. 2010 [54] |
Morphology and attachment of blood components on root surfaces irradiated with Er Cr YSGG laser at different angulations. Laser parameters used were 1.0W, 20hz (140-150 micro seconds), 10% air and 15% water for 30 sec (29.99J/cm2/ pulse) | The Er Cr YSGG irradiated root surfaces proved to be rougher than those scaled with manual instruments; irradiation at working tip angulations of 45° and 60° produced results of attachment of blood components and root wear comparable with those obtained with manual instrumentation. |
| Bolortuya G et al. 2011 [55] |
Comparison of fibroblast cell attachment to 1. Er YAG laser (30 mJ per pulse, 10 pps, 60 s) irradiated dentine vs 2. L-MTAD group: Laser irradiation with application of a mixture of Doxycycline+ Tetracycline + citric Acid treated dentine vs 3. Rc Prep (EDTA gel / cream) vs 4. Control group (no treatment). Cell attachment was observed and evaluated using counting assays and SEM |
The authors observed that the number of cells attached to the laser group was significantly higher than in the Rc Prep and control groups at 16 hours. Also the laser group exhibited dendritic cell extension by fibroblasts as demonstrated by SEM analysis. |
Erbium family of lasers vis a vis conventional methods of root modification.
| S.No | Variables | Conventional methods (Scaling and root planing (SRP) + citric acid/ EDTA/Tetracycline/Fibronectin/Emdogain) |
Erbium family of Lasers |
|---|---|---|---|
| 1. | Calculus removal | Yes | Equivalent to SRP |
| 2. | Preservation of cementum | No | Yes |
| 3. | Removal of endotoxin | Yes | Yes |
| 4. | Smear layer removal | Yes | Yes |
| 5. | pH change on root surface | Yes | No |
| 6. | Thermal damage | No | Minimal |
| 7. | Stable fibrin clot formation | Yes | Yes |
| 8 | Cell attachment | Yes | Better than conventional methods |
| 9. | Cost | Low | High |
| 10. | Patient acceptability | Acceptable | Better than conventional methods |
Erbium lasers are a suitable alternative to conventional methods for root modification.
LASERS AS A THERAPEUTIC MODALITY FOR ROOT SURFACE MODIFICATION
The effect of laser on any given tissue depends on the optical properties of the tissue on which it is incident. The root surface is composed of cementum primarily and in a few cases dentinal tubules exposed at the cemento-enamel junction. Water and hydroxyapatite serve as the chromophores (substance which can absorb the radiation of a particular wavelength). As a result only lasers which are absorbed in these chromophores demonstrate adequate therapeutic ability in root surface modification. These include the erbium family of lasers (Er YAG, Er YSGG), CO2 lasers, Holmium YAG (Yttrium Aluminum Garnet) laser. Among these, the erbium family has shown considerable promise for the purpose of root modification [22].
The Erbium family which includes the erbium YAG (Yttrium Aluminium Garnet) and Erbium chromium YSGG (Yttrium Scandium Gallium Garnet) are solid phase lasers wherein the Yttrium Garnet crystals are doped with Aluminium or Scandium and Gallium. The wavelength of the Erbium YAG is 2940 nm and Er: Cr YSGG 2780 nm respectively. The Erbium: YAG laser belongs to the near infra red spectrum and it has proven efficacy in ablation of dental hard tissue [23, 24].
The unique properties [25] of the Erbium laser family include
- High absorption in water as compared to Carbon dioxide (CO2) / Neodymium Yttrium Aluminium Garnet (Nd:YAG) lasers.
- Good absorption in hydroxyapatite
- Minimal thermal damage to the soft / hard tissue on which it is incident.
The incident laser beam is absorbed by water which undergoes a rapid thermal expansion and induces microexplosions and removal of the hard tissue (enamel, dentin and bone). The erbium family of lasers is used with water (irrigant) and the amount of water flow influences the efficacy and depth of penetration of the laser in the tissue [24].
Methodology for Literature Review
The literature review was performed by conducting a search on PubMed/ Medline using a combination of the MeSH Words “Er: YAG lasers, YSGG Lasers, root scaling, dental calculus, tooth root, ultrasonic therapy”. The available literature was then compiled and the various studies were grouped under the following heads as follows:
Studies evaluating the:
- Efficacy of Erbium family of lasers for calculus removal.
- Root surface changes following Er YAG and Er Cr YSGG application.
- Comparative studies of the Er YAG, Er Cr YSGG lasers versus conventional methods of root surface modification.
- Biocompatibility of root surface following Erbium laser treatment.
- Combined efficacy of laser root modification and conventional methods towards root surface bio-modification.
- Effectiveness of root surface bio-modification prior to root coverage procedures.
CALCULUS REMOVAL FOLLOWING ERBIUM LASER APPLICATION
The Erbium family has been found to be an efficient tool for calculus removal from the root surface with minimal thermal damage to the cementum. This is evidenced in numerous studies which have been summarized in Table 2. In recent times, the removal of calculus has been made more predictable due to the development of a “fluorescence feedback system”. This system was developed based on the findings of a study by Folwaczny et al. [26] who demonstrated a strong fluorescence in sub-gingival calculus when exposed to 655nm diode laser irradiation. This principle has been incorporated into an Er YAG laser unit which gets activated only if a certain threshold level for fluorescence of the root surface is exceeded. From the studies summarized in Table 2, it can be inferred that Erbium laser with fluorescent feedback provided a good alternative to conventional methods for calculus removal.
Root Surface Changes and Parameters Influencing the Root Bio-modification with Erbium Lasers
The Erbium family has a minimum penetration depth due to its high absorption in water. This entails a safe usage with minimum or no thermal damage to the root cementum and dentin. Studies have demonstrated variable changes in the root surface morphology depending on power setting and tip angulations used. Gaspric and Skaleric [38] demonstrated that a variation of energy applied (60mJ, 80 mJ and 100 mJ) resulted in varying root surface morphology. At 60mJ, single craters were produced without deposits of melted mineral and exposed dentinal tubules. At 80mJ, numerous confluent craters were observed. At 100 mJ, larger ablation defects were observed. Er :YAG laser when used at different power settings ranging from 25 to 100 mJ/sec on the root surface in vitro produced a 15 micron layer of damaged tissue within the cementum, with an absence of Sharpey’s fibers [39]. However, when used in vivo a smooth root surface was observed even at higher settings [30].
Angulations of the tip remain a significant factor influencing root surface roughness. Folwaczny et al. [40] evaluated the influence of various tip angulations (15,30,45,60,90 degrees) on root substance removal and surface roughness. A control group consisting of root surface instrumented with curettes was used. The authors observed no statistically significant difference in the roughness values of the root surface treated with laser and curettes. Also the differences in root substance removal were not significant when different angulations were used. The erbium family of lasers appears to be relatively safe at low energy settings and produce minimal root structure damage.
Root Surface Bio-compatibility after Erbium Laser Therapy
Studies have evaluated biocompatibility of the laser treated root surface in terms of
- Elimination of endotoxin from the root surface.
- Removal of the smear layer.
- Reaction of cells to laser treated root surfaces (in vitro).
Endotoxin Elimination from the Root Surface
Endotoxin on the root surface contributes to the cytotoxic effects of the diseased root [4]. An in-vitro study on lipopolysaccharide removal from the root surface with Erbium laser revealed a 83.1% reduction of the lipopolysaccharide from the irradiated root surface [41] The endotoxin removal efficacy of erbium lasers has also been assessed by the chromogenic, quantitative Limulus – amoebocyte-lysate assay. The authors observed a reduction of LPS on root surface irradiated with erbium lasers and this reduction was proportional to the energy setting that is used [42]. Akiyama et al. [43] used SEM and transmission electron microscopy to determine the effect of Er:YAG laser on endotoxin . The authors found that Erbium laser ablates periodontopathic bacteria with thermal vaporization, and its bacterial elimination effect on the diseased root surfaces appears to be superior to that of the ultrasonic scaler. The observations of the above studies provide evidence for the effectiveness of the Erbium laser in managing the cytotoxic changes.
Smear Layer Removal
The formation of a smear layer consisting of dentinal debris and some bacteria occurs after scaling and root planing [8]. The removal of this smear layer assumes clinical importance due to the fact that the smear layer prevents attachment of connective tissue to the root surface. The hard tissue lasers such as the Erbium lasers have been proposed as an alternative to chemical conditioning of root surface for removal of smear layer. Recent studies [44, 45] compared the efficacy of smear layer removal by chemical root modification agents vs Erbium laser (Figs. (1, 2)). The authors have reported an equivalent and complete smear layer removal by erbium laser.

Scanning Electron Microscope picture of periodontally disease root surface treated with citric acid (25%, pH 1.5 for 10 seconds) demonstrating a complete removal of smear layer and patent dentinal tubules.
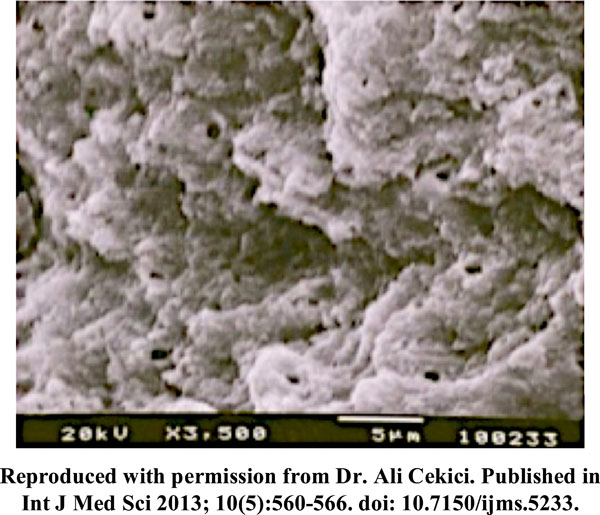
Scanning Electron Microscope picture of periodontally diseased root surface treated with Er YAG laser (15.92 J/cm2) demonstrating the removal of smear layer, roughened root surfaces and patent dentinal tubules.
Biological Reaction of the Cells to Erbium Treated Root Surfaces
This assumes great importance as it represents one of the most important factors influencing success in periodontal regeneration. The various studies performed to assess the biologic reaction of cells of the periodontium to erbium laser treated root surface are summarized in Table 3. It can be inferred from these studies (Table 3) that a stable blood clot is formed and periodontal ligament fibroblast attachment is more favored on the laser treated root surface.
Effectiveness of Combined Therapy in Root Modification
Erbium lasers have also been used in combination with conventional methods such as acid conditioning of the root [56] and recombinant human growth factors (PDGF BB) [57] on periodontally involved root surfaces with improved fibroblast adhesion and proliferation being reported.
Root Surface Bio-Modification Prior to Root Coverage Procedures
Root bio-modification has been performed as an adjunct to root coverage procedures for improving the outcomes. Conventional methods of root bio- modification prior to root coverage include the use of chemical modification agents such as citric acid, tetracycline [58,59]. One of the novel applications of the Er YAG laser has been its use for root bio-modification prior to root coverage procedures. A study by Dilsiz A et al. [60] revealed no significant improvement in the clinical outcomes following adjunctive use of erbium laser for root modification prior to root coverage. A recent systematic review [61] evaluating the effectiveness of adjunctive use of chemical agents and lasers for root bio-modification prior to recession coverage reported no additional benefit in terms of clinical parameter improvement. It can be inferred that erbium lasers do not appear to improve the outcomes of root coverage procedures when used as an adjunct.
A recent systematic review and meta analysis by Sgolatra et al. [62] analyzed the efficacy of Erbium laser as compared to scaling and root planing in treatment of chronic periodontitis. The authors observed no statistically significant difference in any of the investigated clinical parameters and attributed this finding to the heterogeneity of the data collected in the five randomized controlled trials included in the meta analysis.
SUMMARY AND CONCLUSION
The existing literature serves to highlight the promising role of Erbium family of lasers in root bio-modification. The erbium family has a proven anti-bacterial action, predictable calculus removal, minimal root substance removal, and appears to favor cell attachment (when used in combination with acid conditioning or growth factors). A comparative summary of Erbium family of laser vs the conventional techniques for root bio-modification is given in Table 4. In conclusion, Erbium lasers are a useful tool in the periodontist’s armamentarium and can be used for root modification.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
The authors acknowledge the contribution of Ms Cynthia Milton, Lecturer in English, Sri Ramachandra University towards correction of the grammar and language of this manuscript.


