All published articles of this journal are available on ScienceDirect.
Craniofacial Characteristics Related to Daytime Sleepiness Screened by the Paediatric Daytime Sleepiness Scale
Abstract
The present cross-sectional study aimed to assess daytime sleepiness in Chinese adolescents using the Paediatric Daytime Sleepiness Scale (PDSS) and to identify associations between PDSS answers and craniofacial characteristics. A group of 265 Chinese adolescents aged 11-17 years self-completed the PDSS, and their extra- and intra-oral craniofacial characteristics were recorded. Among the participants, 59.7% (157) experienced one or more daytime sleepiness events. No significant associations were found between total PDSS scores and the craniofacial parameters, but when PDSS answers were assessed at the item level, several craniofacial characteristics were found to be positively associated with daytime sleepiness, such as hypertrophic tonsils (P = 0.05), a relatively large tongue (P < 0.01), a bilateral Class II molar relationship (P < 0.05) and increased overjet (P < 0.05). A short lower face (P < 0.01) and a convex profile (P < 0.01) were found to be negatively associated with daytime sleepiness. Daytime sleepiness is commonly reported among Chinese adolescents seeking orthodontic treatment and there are potential associations between the condition and craniofacial characteristics. An assessment of daytime sleepiness is recommended to orthodontists in young patients presenting with hypertrophic tonsils, relative large tongues and Class II tendency malocclusions, and appropriate medical referrals should also be considered.
INTRODUCTION
Daytime sleepiness is common in adolescents. According to a systematic analysis of sleep disturbance among 1,629 Hong Kong adolescents, the prevalence of excessive daytime sleepiness (EDS) was 41.9% [1]. A similar prevalence (41-42%) was reported by a Canadian study based on a sample of 3,235 individuals [2]. Daytime sleepiness can have profound negative effects on the behavior [3], mood [4] and school performance of adolescents [5], and is increasingly becoming a concern.
The main cause of daytime sleepiness in adolescents is inadequate sleep [6], which is influenced by pubertal development [7]. As it is difficult to differentiate insufficient sleep from others causes of daytime sleepiness, such as sleep-disordered breathing (SDB), insomnia, narcolepsy, idiopathic hypersomnia, periodic limb movement during sleep, medication or caffeine intake [8], all causes of daytime sleepiness need to be investigated. Sleep-disordered breathing is experienced during sleep and is caused by prolonged partial obstruction or intermittent complete obstruction of the upper airway, and has been defined as obstructive sleep apnea syndrome (OSAS) with the addition of snoring [9].
Daytime sleepiness is a symptom of SDB, especially in adults with SDB, and was previously thought to be absent in most adolescents [10]. However, this point has been challenged using the gold-standard objective test for daytime sleepiness, i.e., the Multiple Sleep Latency Test (MSLT). Gozal et al. [11, 12] found that 13 to 20% of non-obese adolescents fulfilling the criteria for OSA displayed EDS. As a consensus opinion has not yet been reached [9, 13], the association between paediatric SDB and daytime sleepiness requires additional assessment.
It is hypothesized that if the association between paediatric SDB and daytime sleepiness is strong, then causes of SDB, such as adenotonsillar hypertrophy, obesity and craniofacial anomalies, may be correlated with daytime sleepiness. Adenotonsillar hypertrophy and obesity [14, 15] have been reported as being associated with daytime sleepiness in adolescents. However the question whether craniofacial anomalies are also directly associated with daytime sleepiness remains unanswered. To our knowledge, the present paper is the first paper attempting to associate craniofacial characteristics and daytime sleepiness in Chinese adolescents.
Huynh et al. [16] and Ikävalko et al. [17] have shown orthodontic clinical assessment could offer an important opportunity to evaluate the associate between SDB and craniofacial anomalies; however their samples were Caucasian subjects. In the present study, we utilized orthodontic clinical assessment to investigate the association between the craniofacial characteristics of Chinese adolescents and daytime sleepiness via clinical examination and the Pediatric Daytime Sleepiness Scale (PDSS) questionnaire [18].
MATERIALS AND METHODS
Subjects
A cross-sectional study was conducted at a university teaching hospital. The study was approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (IRB Reference Number: UW 12-405). A sample of Chinese subjects aged 11-17 years seeking orthodontic treatment was recruited. Adolescents with craniofacial syndromes, cleft lips and/or palates or who were evidently overweight (according to their neck and waist circumferences) were excluded.
Based on a previous study [19], the required sample size of the logistic regression with every possible factor considered was at least 150. Given an anticipated dropout rate of 20%, 188 subjects was the minimum sample size.
Data Collection
All of the subjects self-completed a previously validated Chinese version of the eight-item Paediatric Daytime Sleepiness Scale (PDSS) [20]. The craniofacial assessment including several standard extra- and intra-oral examinations was conducted by one trained orthodontist (M.G.) and, in a pilot study, the assessment was calibrated with another experienced orthodontist (Y.Q.Y.).
An extra-oral assessment was performed with the subject sitting upright and in Natural Head Position (NHP), and included assessment of the profile and frontal views and the mandibular plane angle [21, 22] (Table 1).
Details of craniofacial assessment.
| Extra-oral | ||
|---|---|---|
| The profile view | Convex / straight / concave | Convex when a line dropped from the forehead to the base of the upper lip and a second line extending from that point downward to the soft-tissue pogonion formed an acute angle; straight when the angle was a nearly straight line and concave when the angle was obtuse |
| The Frontal view | Short / normal / long lower face | Short lower face when the lower third of the face was shorter than average; normal lower face when the lower third was almost equal to the average and long lower face when the lower third was longer than average. The mid-third of the face was used as a reference |
| The mandiular plane angle | Flat / normal / steep | Visualized by placing a mirror handle along the border of the mandible, classified as flat, normal or steep. |
| Intra-oral soft tissue | ||
| Tonsil size | Normal / hyprtrophic | Normal or hypertrophic when the tonsils caused a greater than 50% obstruction, according to the Brodsky grade |
| Relative tongue size | Normal / large | Normal or large when imprints of the teeth were seen on the lateral margins of the tongue, which is relative tongue size in realtion to the dental arch, rather than a macroglossia |
| Intra-oral dentition | ||
| Shape of upper arch | Tapered / ovoid / square | According to the MBT system |
| Shape of lower arch | Tapered / ovoid / square | According to the MBT system |
| Height of palatal vault | High / Normal | The palatal vault was classied as high (deep, constricted, V-shaped palatal arch) and normal (Shallow, U-shaped palatal arch) |
| Molar relationship | Class I / Class II / Class II subdivison / Class III / Class III subdivision | According to the Angle classification, in which the subdivision is the unilateral molar relationship of either Class II or Class III |
| Incisor relationship | Class I / Class II subdivison 1 / Class II subdivison 2 / Class III | According to the British standard incisor classification |
| Vertical occlusion | Reduced overbite / normal / deepbite / reverse overbie | A visual occlusion assessment |
| Amout of overjet | A linear measurement with a ruler |
Profile of subjects recruited in present study.
| Category | Number | |
|---|---|---|
| N | Total: 265 | |
| Age | Mean ± SD: 13.7 ± 1.9 | |
| Range: 11 - 17 | ||
| Overjet | Mean ± SD: 3.9 ± 3.7 | |
| Range: -7.0 - 15.0 | ||
| BMI | Mean ± SD: 19.1 ± 3.2 | |
| Range: 13.1 - 31.6 | ||
| Normal | 245 (93.9%) | |
| Overweight | 16 (6.1%) | |
| Sex | Female | 166 (62.6%) |
| Male | 99 (37.4%) | |
| Profile view | Convex | 179 (67.5%) |
| Straight | 52 (19.7%) | |
| Concave | 34 (12.8%) | |
| Frontal view | Short lower face | 54 (20.4%) |
| Normal lower face | 140 (52.8%) | |
| Long lower face | 71 (26.8%) | |
| Mandibular angle | Flat | 43 (16.2%) |
| Normal | 125 (47.2%) | |
| Steep | 97 (36.6%) | |
| Tonsil size | Normal | 244 (92.8%) |
| Hypertrophic | 19 (7.2%) | |
| Relative tongue size | Normal | 241 (91.6%) |
| Large | 22 (8.4%) | |
| Shape of upper arch | Tapered | 36 (13.6%) |
| Ovoid | 175 (66.3%) | |
| Square | 53 (20.1%) | |
| Shape of lower arch | Tapered | 40 (15.1%) |
| Ovoid | 184 (69.4%) | |
| Square | 41 (15.5%) | |
| Height of palatal vault | High | 55 (21.1%) |
| Normal | 206 (78.8%) | |
| Molar relationship | Class I | 95 (35.9%) |
| ClassII | 59 (22.3%) | |
| ClassII subdivision | 43(16.2%) | |
| ClassIII | 39 (14.7%) | |
| ClassIII subdivision | 29(10.9%) | |
| Incisor relationship | ClassI | 73 (28.3%) |
| ClassII division1 | 121 (46.9%) | |
| ClassII division2 | 13 (5.0%) | |
| ClassIII | 51 (19.8%) | |
| Vertical occlusion | Reduced overbite | 28 (10.8%) |
| Normal | 115 (44.4%) | |
| Deepbite | 85 (32.8%) | |
| Reverse overbite | 31 (12%) | |
Responses to the PDSS questions.
| Events "sometimes/seldom/never" | Events "always/frequently" | |
|---|---|---|
| PDSS score | Mean ± SD: 12.2 ± 4.5 | |
| Range: 0 - 28 | ||
| Sum answers of Q1-Q8 | 106 (40.3%) | 157 (59.7%) |
| Answer to Q1: "fall asleep or get drowsy during class periods" | 240 (90.6%) | 25 (9.4%) |
| Answer to Q2: "get sleepy or drowsy while doing your homework" | 243 (91.7%) | 22 (8.3%) |
| *Answer to Q3: "usually alert most of the day" | 16 (6.0%) | 249 (94.0%) |
| Answer to Q4: "ever tired and grumpy during the day" | 236 (89.4%) | 28 (10.6%) |
| Answer to Q5: "have trouble getting out tobed in the morning" | 215 (81.1%) | 50 (18.9%) |
| Answer to Q6: "fall back to sleep after being awakened in the morning" | 235 (88.7%) | 30 (11.3%) |
| Answer to Q7: "need someone to awaken you in the morning" | 168 (63.6%) | 96 (36.4%) |
| Answer to Q8: "you need more sleep" | 180 (67.9%) | 85 (36.4%) |
* The events of daytime sleepiness should be reversed for the answer to Q3.
Statistical association between the frontal view and PDSS answers.
| PDSS item | Convex Profile (67.5%) | Concave and Staight Profile (32.5%) | P value | |
|---|---|---|---|---|
| PDSS answer to Q1 | Events "sometimes/seldom/never" | 168 (93.9%) | 72 (83.7%) | |
| Events "always/frequently" | 11 (6.1%) | 14 (16.3%) | 0.008** | |
| PDSS answer to Q4 | Events "sometimes/seldom/never" | 165 (92.2%) | 71 (83.5%) | |
| Events "always/frequently" | 14 (7.8%) | 14 (16.8%) | 0.033* | |
*P<0.05; **P<0.01;
Statistical association between the profile view and PDSS answers.
| PDSS item | Short lower face (20.4%) | Normal and Long Lower Face (79.6%) | P value | |
|---|---|---|---|---|
| PDSS answer to Q7 | Events "sometimes/seldom/never" | 41 (75.9%) | 127 (60.5%) | |
| Events "always/frequently" | 13 (24.1%) | 83 (39.5%) | 0.035* | |
* P<0.05;
Statistical association between the relative tongue size and PDSS answers.
| PDSS item | Small and normal tongue (91.6%) | Large tongue (8.4%) | P value | |
|---|---|---|---|---|
| PDSS answer to Q5 | Events "sometimes/seldom/never" | 201 (83.4%) | 12 (54.5%) | |
| Events "always/frequently" | 40 (16.6%) | 10 (45.5%) | 0.001*** | |
***P<0.001
Statistical association between the molar relationships and PDSS answers.
| PDSS item | Other Molar Relationships (77.7%) | Class II molar (22.3%) | P value | |
|---|---|---|---|---|
| PDSS answer to Q6 | Events "sometimes/seldom/never" | 192 (91.4%) | 43 (78.2%) | |
| Events "always/frequently" | 18 (6.8%) | 12 (21.8%) | 0.006** | |
**P<0.01;
Statistical association between the overjet and PDSS answers.
| PDSS item | Overjet (Mean ± SD) | P value | |
|---|---|---|---|
| PDSS answer to Q6 | Events "sometimes/seldom/never" | 3.7 ± 3.6 | |
| Events "always/frequently" | 5.1 ± 3.7 | 0.037* | |
*P<0.05;
Association between craniofacial variables and PDSS answers shown by binary logistic regression.
| B | S.E. | OR | OR (95%CI) | P value | |
|---|---|---|---|---|---|
| Q1: Straight or concave profile vs. convex profile | 1.0 | 0.4 | 2.7 | 1.2-6.4 | 0.018* |
| Q4: Straight or concave profile vs. convex profile | 0.8 | 0.4 | 2.3 | 1.0-5.2 | 0.040* |
| Q5: Hypertrophy tonsil size vs. normal | 1.1 | 0.6 | 3.0 | 1.0-9.0 | 0.050* |
| Q5: Large tongue size vs. normal | 1.8 | 0.5 | 5.9 | 2.2-15.6 | <0.001*** |
| Q6: Class II molar relationship vs. other molar relastionships | 0.9 | 0.4 | 2.4 | 1.0-5.5 | 0.047* |
| Q7: Younger age vs. older | 0.3 | 0.1 | 1.3 | 1.1-1.5 | 0.001*** |
| Q7: Normal and long lower face vs. short lower face | 0.9 | 0.4 | 2.6 | 1.2-5.3 | 0.011* |
| Q8: Large tongue size vs. normal | 1.0 | 0.5 | 2.7 | 1.0-6.8 | 0.043* |
*P<0.05; ***P<0.001
B: estimated logit coefficient; S.E.: standard error of the coefficient; OR: odds ratio; CI: confidence interval.
The intra-oral soft tissue was evaluated according to the subject’s tonsil size (according to the Brodsky grade) [23]; and relative tongue size (Fig. 1) [24] (Table 1).
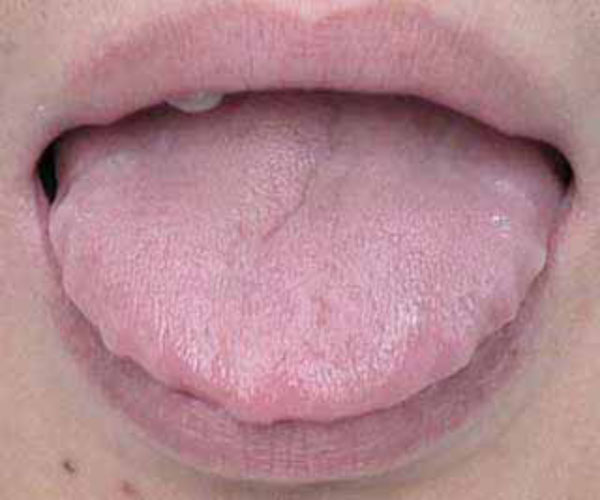
Illustration of a relatively large tongue (see the imprints of the lateral margins of the tongue).
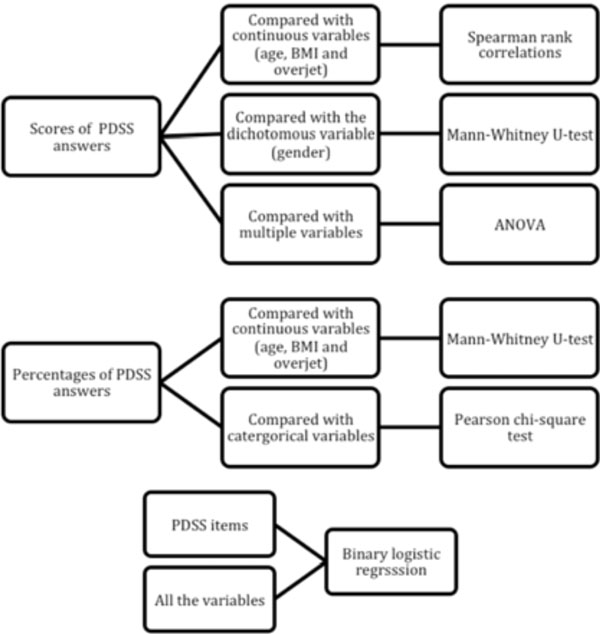
Flowchart of statistical tests used in the present study Table 2. Profile of subjects recruited in the present study.
The dentition was assessed according to the shape of the upper arch, and lower arch, the height of palatal vault, the molar relationship, the incisor relationship, the overbite [25-27] and the amount of overjet (Table 1).
The subjects’ basic demographics were also recorded, including age, sex and body mass index (BMI).
DATA ANALYSIS
Data are presented as the mean, standard deviation and range for the continuous variables and as percentages for the categorical variables. Cronbach’s alpha was used to assess the internal consistency of the answers to the eight-item PDSS questions. The total PDSS scores were compared with continuous variables (age, BMI and overjet) using Spearman rank correlations; with the dichotomous variable (Insex) using the Mann-Whitney U-test and with the other multiple variables using Kruskal-Wallis one-way analysis of variance (ANOVA). Responses to the PDSS were also analyzed as percentages by combining the events “always or frequently” into “yes” and “sometimes, seldom and never” into “no” for each individual item. These data were compared with continuous variables (age, BMI and overjet) using the Mann-Whitney U-test and with categorical variables using the Pearson chi-square test. A binary logistic regression was calculated to assess the associations between each PDSS item and all of the variables. Statistical significance was set at a P < 0.05. Statistical analyses were performed with SPSS software (IBM SPSS Statistics 20, IBM Corp.) (Fig. 2).
RESULTS
Subject Profiles and PDSS Response Rate
Of the subjects who sought orthodontic treatment during the 2012 screening sessions, 265 subjects (99 boys and 166 girls; mean age 13.7 ± 1.9 years) met the inclusive criteria and agreed to complete the questionnaire undergo a clinical examination. The subject profiles and response rates are presented in Tables 2 and 3.
The Cronbach’s alpha of the PDSS was 0.7, indicating satisfactory internal consistency. The PDSS scores ranged from 0 to 28 (mean ± SD, 12.2 ± 4.5). Though the reported daytime sleepiness events for each individual item were considerably low, the sum of the items showed that 59.7% of the subjects reported “yes” for the daytime sleepiness event.
Association Between the PDSS and Craniofacial Variables
The statistical analysis showed no significant associations between the total PDSS scores and the craniofacial variables. In the evaluation of the PDSS answers as categorical data at the item level, chi-square analysis showed that the subjects classified as having a convex profile (P = 0.008 for Q1 and P = 0.033 for Q4) (Table 4) or a short lower face (P = 0.035 for Q7) (Table 5) experienced less daytime sleepiness than the other subjects. In contrast, subjects with a relatively large tongue (P = 0.001 in Q5) (Table 6) and a bilateral Class II molar relationship (P = 0.006 in Q6) (Table 7) experienced more daytime sleepiness than the other subjects. In addition, the Mann-Whitney U-test showed more daytime sleepiness was associated with a larger overjet (P = 0.037 for Q6, 5.1 ± 5.7 vs. 3.7 ± 3.6 mm) (Table 8). Although a statistical association between age (P = 0.008 in Q7) and daytimesleepiness events was also shown by Mann-Whitney U-test, the actual age difference was very minimal (13.9 ± 2.0 vs. 13.3 ± 1.9 years). Binary logistic regression analysis showed similar results, in which hypertrophic tonsils, a relatively large tongue and a bilateral Class II molar relationship were positively associated with daytime sleepiness, and that a short lower face, convex profile and age were negatively associated with daytime sleepiness (Table 9).
DISCUSSION
The Rationale of the Present Study
To associate daytime sleepiness with craniofacial anomalies, it would have been optimal to assess the former using the gold-standard objective test (i.e., the MSLT) and the latter using a lateral cephalogram or three-dimensional image analysis (CT or MRI). However, in the case of our orthodontic screening subjects, it was not possible to diagnose daytime sleepiness using the MSLT due to time and cost limitations, and for ethical reasons, no radiographs were taken.
The use of questionnaires to identify daytime sleepiness in adolescents with suspected SDB was found to be effective. Chervin et al. [14] reported that subjective sleepiness can be elicited by the Paediatric Sleep Questionnaire (PSQ), and the validity of their findings was verified by the MSLT. Melendres et al. [28] found that adolescents with suspected SDB showed more daytime sleepiness than control subjects according to the modified Epworth Sleepiness Scale (ESS), and the ESS was found to be correlated with the polysomnographic parameters. Drake et al. [18] reported the PDSS was suitable for the assessment of daytime sleepiness for middle-school-age adolescents, and its Chinese version which was used in the present study, was also reported as a reliable and valid tool for the measurement of sleepiness and for the screening for more severe pathological sleepiness, such as narcoplepsy [20].
The PDSS was chosen in the present study because the adolescent subjects were in the middle-school-age, and the self-completed questionnaire was considered to be more precise than a parent-reported questionnaire [29]. The limitations of PDSS were its lack of validation against the MSLT, and in the present study, its validity was not compared to the other parent-reported or teacher-reported questionnaires.
The most common cause of daytime sleepiness in adolescents is insufficient sleep, which is affected by pubertal development [7]. Previous studies have shown that pubertal development is associated with an increase in daytime sleepiness and post-pubertal adolescents may need more sleep to maintain pre-pubertal levels of daytime alertness [30, 31]. Therefore the direct association between daytime sleepiness and craniofacial anomalies is likely to be subtle. The present study used the score-based and item-based methods to perform the analysis. Another commonly used questionnaire (PSQ) was also applied via either a score-based or item-based analysis [14, 15]. As the value of the Cronbach’s alpha in the present study showed satisfactory internal consistency (0.7), the item-based analysis was considered reliable.
The Prevalence of Daytime Sleepiness Found in the Present Study
The present study showed that, although reported daytime sleepiness events were not frequent for the individual questions (from 6% to 36.4%), a high incidence of events (59.7%) was found for the summed items. This indicated that a high proportion of Chinese adolescents seeking orthodontic treatment were experiencing one or more daytime sleepiness events. This number (59.7%) was higher than the reported prevalence of daytime sleepiness (41.9%) of the general adolescent population in Hong Kong [1], implying that adolescents with craniofacial anomalies or malocclusion are experiencing more daytime sleepiness than the general population. Although this conclusion was not based on rigorous investigation, it reflected a trend. Since the 265 subjects were selected from the only teaching/public dental hospital in Hong Kong and the screening clinics were conducted for a year (2012), they could be considered to represent the total Chinese adolescent orthodontic population.
The Association Between Craniofacial Characteristics and Daytime Sleepiness
When PDSS responses were assessed at the item level, several craniofacial features were found to be associated with daytime sleepiness. Hypertrophic tonsils and a relatively large tongue were associated with a high risk of daytime sleepiness and a Class II tendency including increased overjet and a bilateral Class II molar relationship was also positively related. Adolescents with a short lower face and convex profile were found to be at low risk of daytime sleepiness. However it should be appreciated that the bilateral class II molar relationship may not be indicative of the under lying skeletal pattern due to early loss of deciduous teeth and molar drifting.
Hypertrophy of the tonsils and adenoids is a widely acknowledged high risk factor for SDB [32, 33]. Although the size of the tonsils of this adolescent population (aged 11-17 years) should have begun to reduce from their maximum size during the pre-pubertal years, the results show that hypertrophic tonsils were found to be associated with a high risk of daytime sleepiness.
The present study found a relatively large tongue size was associated with daytime sleepiness, however Arens et al. [34] reported a similar tongue size of children suffering from OSAS and a normal control group. This difference may be explained by age, as the mean age of the subjects in the study by Arens et al. was 4.8 years, compared with 13.7 years in the present study. Considering that tongue size is a confirmed risk factor for adult OSAS [35, 36], this may imply that the tongue is associated with the daytime sleepiness as a result of increasing age.
As no previous study has focused on the association between craniofacial characteristics and daytime sleepiness, the results of the present study could only be compared with the relationship between craniofacial characteristics and SDB.
It was found that a Class II tendency, including increased overjet and a bilateral Class II molar relationship, was associated with daytime sleepiness. A similar study based on dental models also reported an association between increased overjet, Class II molar relationship and SDB [37]. Two other recent meta-analyses also have reported a relationship between a Class II tendency and paediatric SDB [38, 39]. Flores-Mir et al. [38] identified that the SNB and ANB angles in children with OSAS had statistically significant differences in comparison with those in a control group. Katyal et al. [39] found that children with OSAS and primary snoring showed increases of 1.64° and 1.54° in the ANB angle compared with the controls, respectively.
According to the result of the present study, the unilateral Class II molar relationship (e.g., Class II subdivision) may not have the same association as the bilateral molar relationship; because the unilateral Class II molar relationship is caused by the mesial migration of an upper molar in a Class I skeletal base rather an actual Class II skeletal relationship.
Two similar studies reported an association between the profile and SDB. Huynh et al. [16] reported that a dolichofacial (long face) is related to SDB. Although the present study did not find subjects with a long lower face to be at risk of daytime sleepiness, subjects with a short lower face were found to be at low risk of daytime sleepiness. Ikävalko et al. [17] reported that a convex profile was a high risk factor for SDB. On the contrary, the present study found a convex profile was at lower risk of daytime sleepiness compared with those with a concave or a straight profile. Possible reasons include differences in age of the subjects; 6-8 years old compared with 11-17 years old in the present study and secondly, ethnicity. Compared with Caucasians, more Chinese people with a convex profile have a Class I occlusion rather than Class II [40].
The associations between daytime sleepiness and the three demographic variables examined (age, sex and BMI) were also analyzed via binary logistic regression. Since obesity was a confounding factor, obviously overweight subjects were excluded. Only 16 (6.1%) subjects were classified as overweight according to the BMI cutoff score of the International Obesity Task Force [41], and no association was found between daytime sleepiness and BMI in the study population. Furthermore, no association was found between daytime sleepiness and sex in the present study. Carskadon et al. [30] also reported no sex difference between pubertal subjects with daytime sleepiness. However, greater daytime sleepiness was found in older subjects, which was not confirmed by the present study.
The Implications to Orthodontists
In the present study, when the PDSS answers were analyzed along with the scores, no associations were found. Thus, we believe that the craniofacial parameters investigated in this study are only potentially associated with daytime sleepiness, and further studies are required. Although the present study focused on Chinese adolescents, a similar association between craniofacial anomalies and SDB was reported for Caucasian adolescents. Therefore in order to decrease the potential impact of daytime sleepiness on adolescents’ health, an assessment of daytime sleepiness is recommended to orthodontists when examining young patients presenting with hypertrophic tonsils, relatively large tongues and Class II tendency malocclusions. Appropriate medical referrals should also be considered.
CONCLUSION
Daytime sleepiness is commonly reported among Chinese adolescents seeking orthodontic treatment. A relatively large tongue, hypertrophic tonsils and a Class II tendency (including increased overjet and a bilateral Class II molar relationship) are potential contributing factors to daytime sleepiness. An assessment for daytime sleepiness is recommended to orthodontists in this group of patients.
CONFLICT OF INTEREST
The authors declare that this study was funded by the research funding of the University of Hong Kong, and we have no financial and non-financial competing interest.
ACKNOWLEDGEMENTS
The authors sincerely thank Kar Yan Li for statistical assistance and Yu-Shu Huang (Chang Gung Memorial University Hospital, Taipei) and Daniel K. Ng (Kwong Wah Hospital, Hong Kong SAR) for kindly providing the Chinese version of the PDSS.


