All published articles of this journal are available on ScienceDirect.
Central Odontogenic Fibroma of the Gingiva: A Case Report
Abstract
In this paper, we present a case of an uncommon and slow-growing tumor known as a central odontogenic fibroma (COF). The patient in question is a 53-year-old African-American man who was referred for periodontal evaluation of asymptomatic space formation between the mandibular central incisors. Clinical and radiological evaluations disclosed tumor-like tissue expanding the alveolar ridge in the buccolingual dimension, along with thinning of the cortical plates. Surgical excision was performed, and the specimen was sent for histopathology, which later confirmed that the lesion was a COF. Periodontal regenerative therapy was performed to rebuild the hard and soft tissue that had been compromised as a result of tumor expansion. The site was grafted, with excellent results.
INTRODUCTION
Central odontogenic fibroma (COF) is an extremely rare, benign tumor with many cases being documented in women [1]. However, the current patient is male, thereby contributing to the rarity of this case.
COF is derived from dental mesenchymal tissue. The tumor is asymptomatic and may evolve from either a dental germ (dental papilla or follicle) or from the periodontal membrane. It is therefore related to the radical or coronal portion of teeth. To date, only 70 cases have been documented, such that COF constitutes 0.1% of all odontogenic tumors. It is so rare that age and sex distribution, as well as typical location cannot be accurately determined [2]. Because of this, there is little reliable data to support its occurrence [1].
Gardner [3] further sought to clarify lesions previously diagnosed as COF. He defined two histological variants: simple and complex (World Health Organization [WHO] type). Simple COF is characterized by a hyperplastic dental follicle with connective fibrous tissue and small amounts of odontogenic epithelium. Complex COF is composed of connective cellular tissue, a prominent epithelial component, and the presence of variable quantities of dentin or cementum-like tissue. The complex type, mainly found in the mandible, is usually more aggressive, provoking expansion of cortical bone, paresthesia of the inferior dental nerve, and pain. Granular cells are apparent in ameloblastoma, ameloblastic fibroma, COF, and numerous other tumors. As a result, the accurate diagnosis of COF is particularly difficult [1]. The WHO classifies odontogenic fibroma as (a) the WHO variant or (b) the non-WHO variant. The WHO variant is considered a mesenchymal odontogenic tumor and comprises two distinct cell types: a fibrous element, and an epithelial component that resembles dental lamina or its remnants. On the other hand, the non-WHO variant lacks an epithelial component and is a monomorphic fibroblastic tumor, purported to be of odontogenic mesenchymal origin, and ostensibly derived from pulpal or follicular fibroblasts [4].
CASE REPORT
A 53-year-old African-American man was referred to the clinic by his general dentist for periodontal evaluation of space formation between the mandibular central incisors (teeth 24 and 25). This spacing was first brought to attention by the patient’s sister. The patient did not complain of any pain, swelling, or bleeding from the site and had no additional symptoms or concerns. His past medical and surgical history was non-contributory.
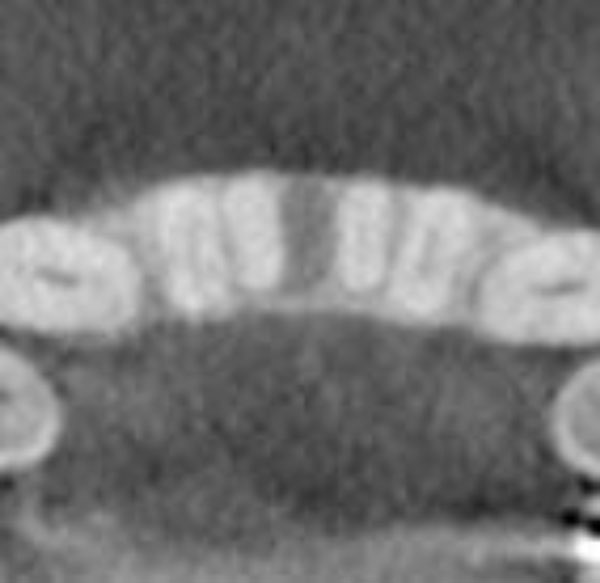
Clinical examination revealed the space formation between teeth 24 and 25 (Fig. 1a). The overlying mucosa and gingiva appeared normal in color. Deep probing elicited bleeding from the problem site. Radiographic evaluation revealed a well-circumscribed, low-density, radiolucent lesion between the mandibular central incisors, with spacing between teeth 24 and 25 (Fig. 1b) as well as thinning and expansion of the buccal and lingual cortical plates (Figs. 1c-1e). No other pathology was detected by the oral and maxillofacial radiologist on the images.
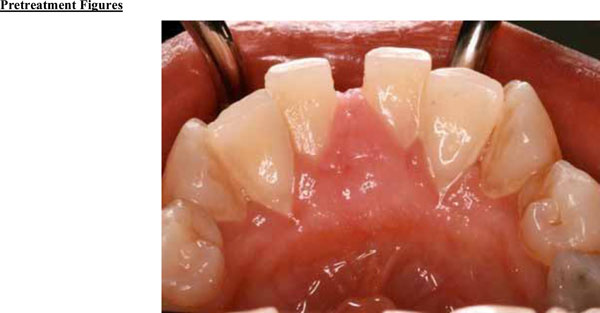
Expansion of the lingual aspect in the anterior mandible.
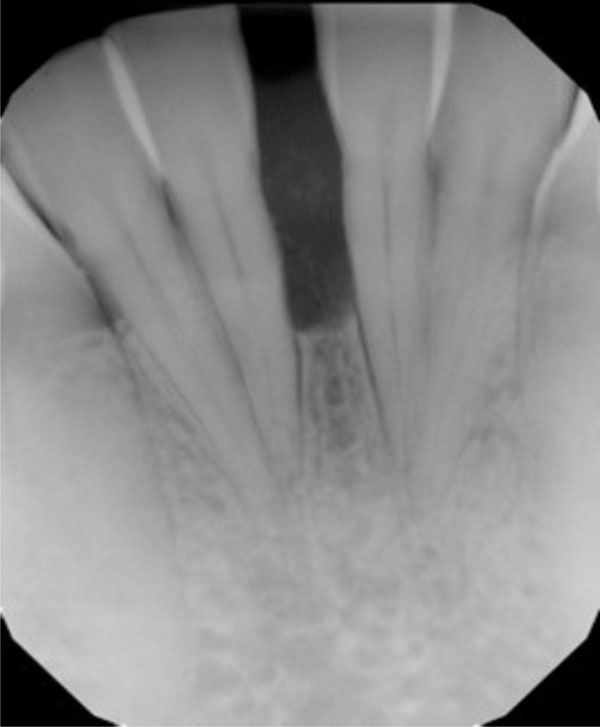
Radiograph suggests vertical bone loss and separation of teeth 24 and 25.
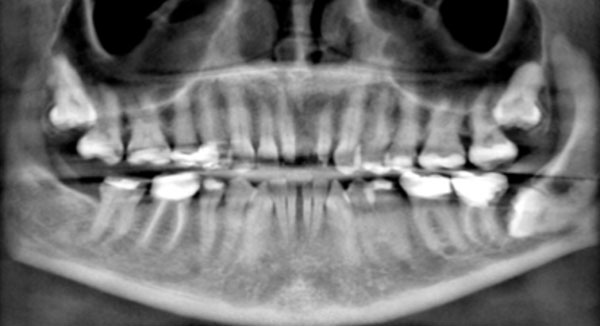
Reconstructed panoramic radiograph shows impacted teeth, missing teeth, and migration of teeth. The mandibular left third molar is horizontally impacted against the distal surface of the second molar. The root of the molar appears to be irregular, potentially suggesting root resorption.
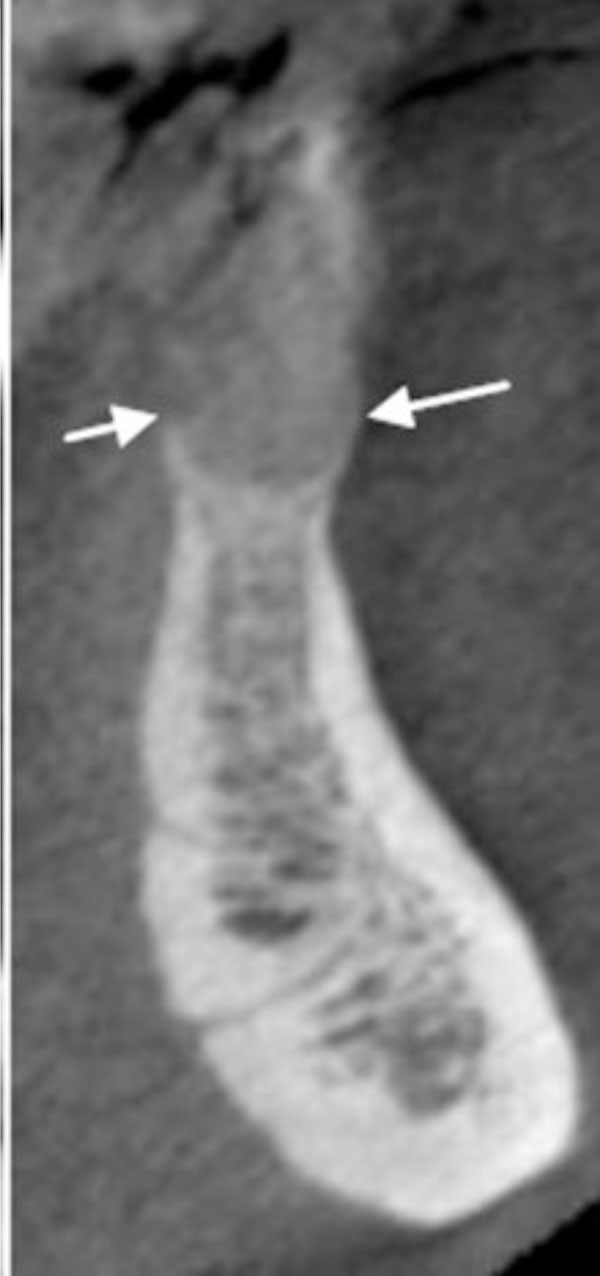
Three-dimensional image showing the loss of vertical dimension and buccolingual expansion of the alveolar bone.
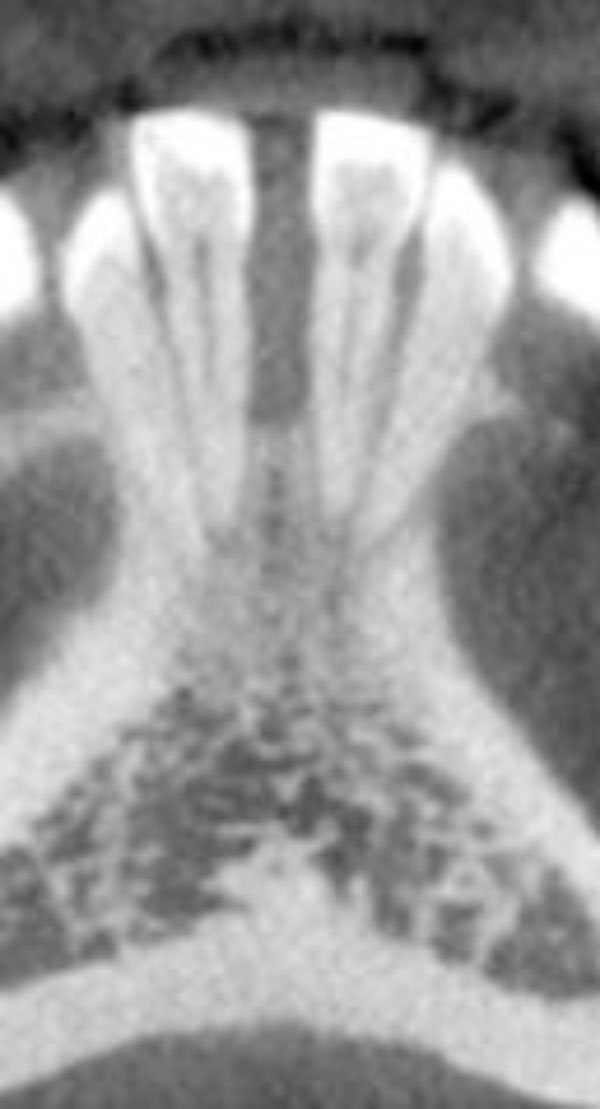
Severe vertical bone loss is evident on this image of mandibular central incisors.
The buccal and lingual cortical plates appear to be thinned and expanded. A well-circumscribed radiolucent lesion is present between the two mandibular central incisors.
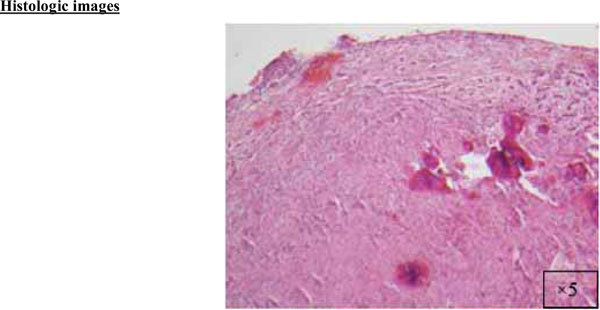
A cellular fibroblastic connective tissue containing numerous cords and nests of odontogenic epithelium (x5).
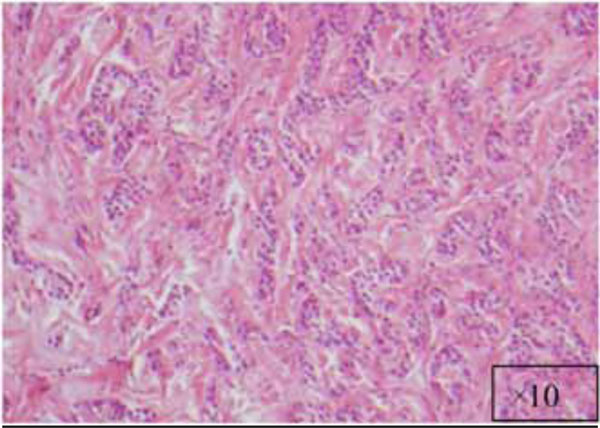
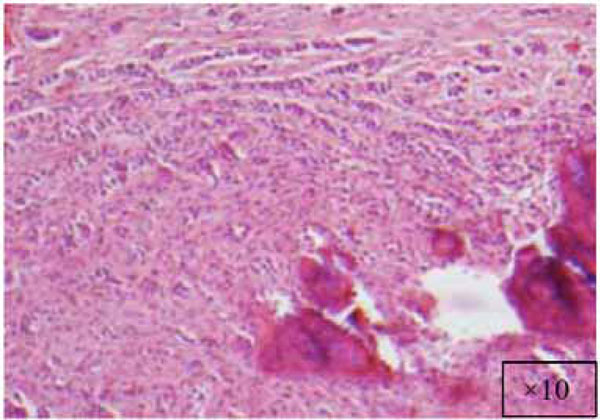
The lesion at a higher magnification (x10).
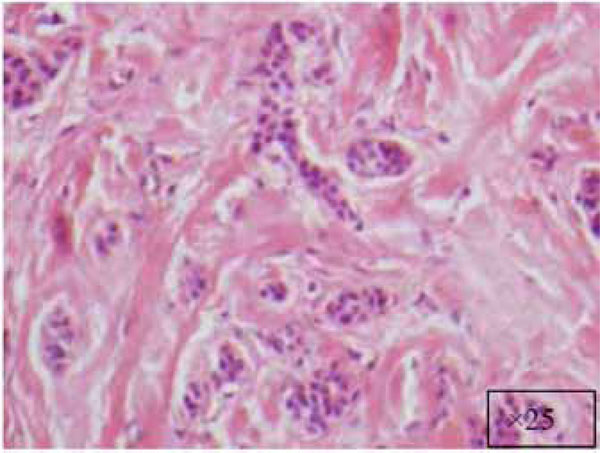
At a higher magnification (x25).
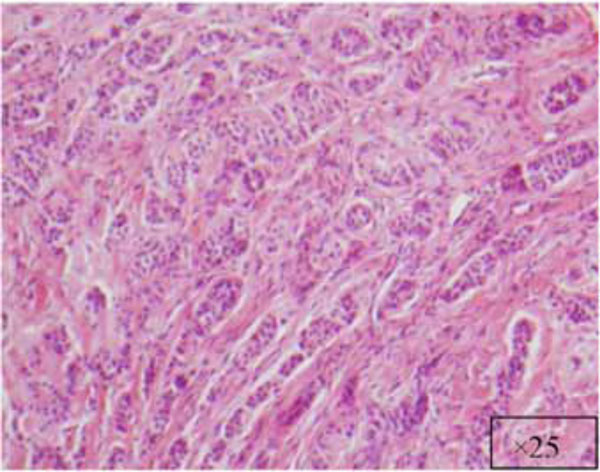
Another view at x25.
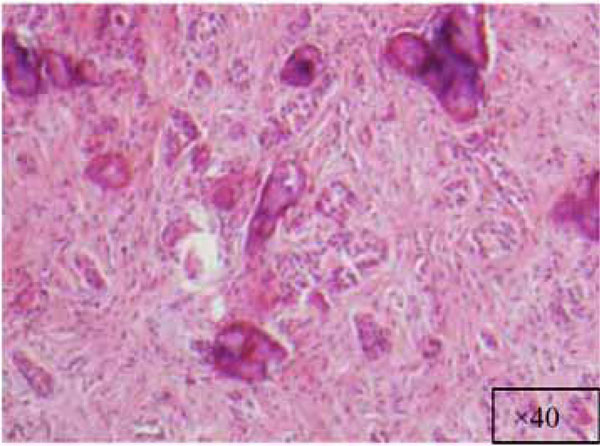
Higher magnification of the lesion (x40).
A cellular fibroblastic lesion containing numerous cords and nests of odontogenic epithelium, and dysplastic dentin or cementumlike calcification material (x10).
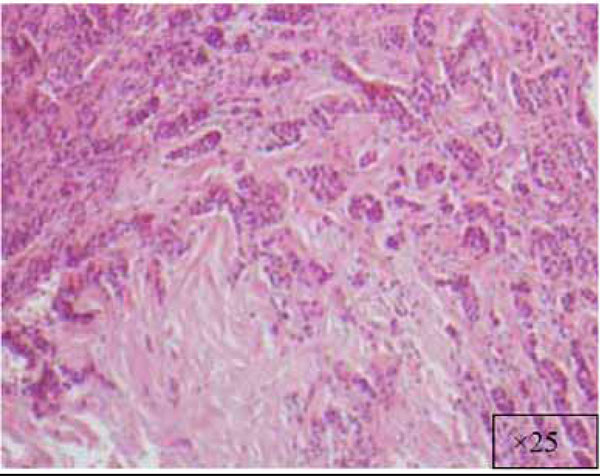
View at x25.
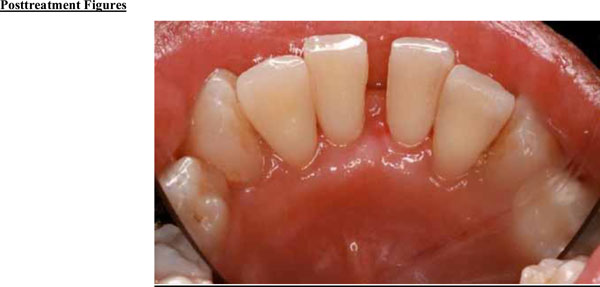
Significant improvements in tissue color, contour, and consistency are apparent at 6 months after removal of the fibroma.
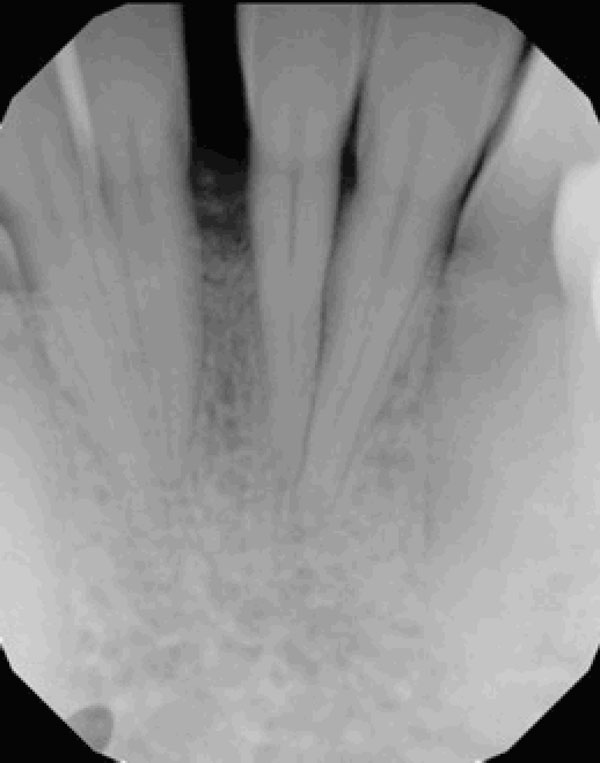
Significant improvement in bone quantity and a reduction in the separation of teeth 24 and 25 are evident on the 6-month posttreatment radiograph.
The findings and treatment plan were discussed with the patient, who agreed to proceed with treatment. A surgical incisional biopsy specimen (1.3 × 1.1 × 0.2 cm) was obtained, removed, and sent for histopathological evaluation. Images from the current patient’s biopsy specimens are shown in Figs. (2a-2g). The specimen was made up of a cellular fibroblastic connective tissue containing numerous cords and nests of odontogenic epithelium and dysplastic dentin or cementum-like calcification material. The mass was diagnosed as COF, WHO type, because of the presence of fibrous and epithelial components.
The tumor was successfully removed, and to rebuild the hard and soft tissue that had been compromised as a result of tumor expansion, periodontal regenerative therapy was offered. The patient accepted the therapy and the surgical site was bone grafted, with excellent results (Figs. 3a and 3b). Preventive measures were taken before the tumor was able to spread further, which might have led to severe bone loss and possible complications.
DISCUSSION
COF tends to have a female-to-male predominance of 3:1, with peak incidence in the 2nd to 4th decades. Svirsky et al. [5] analyzed 15 cases of COF and reported that 80% occurred in the mandible. In a study carried out by Handlers et al. [6], of the 39 cases of COF found, 56% were reported to have occurred in the maxilla and 44% in the mandible. The most recent review of the literature by Ramer et al. [7] reported a similar incidence in the maxilla and mandible.
Clinical, Histopathological, and Radiographic Appearance
Clinically, COF tends to manifest as an asymptomatic swelling, although it can appear in a more aggressive way, provoking dental displacement and rhizolysis [1]. The current patient’s lesion was central in the jaws and exhibited a slow persistent growth that resulted in painless cortical expansion. COF is usually a painless mass. The tumor occurs more frequently in the anterior maxilla and may result in a distinctive palatal cleft or depression, which is considered characteristic of this tumor. Large lesions may affect adjacent structures, move teeth (as evident in our case), or even cause root resorption [2]. Specimens are usually encapsulated, firm, smooth masses. Surgeons may describe the lesion as “shelled out” or hollowed out [8].
Histologically, COF is defined as a fibroplastic neoplastic lesion that contains inactive odontogenic epithelium and variable quantities of calcified material. COF may be associated with the crown of an included tooth or the roots of erupted teeth. The maxilla shows a predilection for the front part of the mouth, whereas in the mandible there is a predilection for the posterior regions [1]. Due to its non-exclusive histological features, this lesion may be confused with other entities, such as hyperplastic dental follicles, odontogenic myxomas, and desmoplastic fibromas, highlighting the importance of clinicopathological correlation in the diagnosis of COF [2]. Careful diagnosis is crucial.
There is a wide variety of histologic appearances, ranging from densely hyalinized and cellular, to loose and myxomatous, to nearly acellular (see Fig. 2). Delicate collagen fibers are occasionally identified, along with fibromyxoid stroma. It is this variation that has resulted in the historical separation of COF into two types: the epithelium-poor type (formally referred to as the simple type) or the epithelium-rich type (formally referred to as the WHO type). Features of odontogenic fibromas include inactive-looking odontogenic epithelium that, when present, may appear proliferative or form irregular islands and cords. Calcifications may or may not be present, simulating cementum, osteoid, or dentin. A rare granular cell odontogenic fibroma variant also exists [8].
Radiologically, COF manifests as a unilocular or multilocular lesion with well-defined margins and surrounded by a sclerotic halo. The multilocular radiotransparent form is more frequently associated with complications such as severe resorption of the roots of adjacent teeth, displacement of neighboring teeth, or inclusion of some tooth tissue [1]. Severe effects on the surrounding bone trabeculae are often seen, even if the borders are well defined on radiographic images. Kaffe et al. [9] stated that a differential diagnosis of COF should be considered for all abnormal radiolucencies in the jaws, as a diagnosis of COF can be difficult for a variety of reasons. Ikeshima and Utsumomiya [10] attempted to show specific characteristics of fibromas for differential diagnosis, such as boundaries, root resorption and calcification. Other reports have noted that COFs are multilocular, radiolucent, slowly growing neoplasms of bone with no tendency to recur after surgical enucleation and have a variety of radiographic and histopathological characteristics.
Differential Diagnosis
Wesley et al. in 1975 [11] suggested a set of criteria for diagnosing COF:
1) Clinically, the lesion is central in the jaws and has a slow, persistent growth that results in painless cortical expansion.
2) Radiologically, the appearance of the lesion varies, but like ameloblastoma and odontogenic myxoma, most are multilocular radiolucent lesions that involve relatively large portions of the jaws in the later stages. In some instances, they may be associated with unerupted and/or displaced teeth.
3) Histopathologically, the most consistent feature is a tumor composed predominantly of mature collagen fibers with numerous interspersed fibroblasts. The presence of small nests and/or strands of inactive odontogenic epithelium is a variable feature.
4) The lesion is benign and responds well to surgical enucleation, with no tendency to undergo malignant transformation.
Gardner [3] attempted further clarification of lesions previously described as odontogenic fibroma and classified them into three different, yet probably related, lesions:
1) The hyperplastic dental follicle.
2) A fibrous neoplasm with varying collagenous fibrous connective tissue containing nests of odontogenic epithelium (simple type).
3) A more complicated lesion with features of dysplastic dentin or cementum-like tissue and varying amounts of odontogenic epithelium (WHO type). As this group is similar to the calcifying odontogenic tumor described by Pindborg in a WHO publication in 1971 [2, 12], Gardner designated it as odontogenic fibroma (WHO type) [3]. The two lesions can be distinguished by the fact that, the calcifying odontogenic tumor stains positive with amyloid stains, but the odontogenic fibroma (WHO type) does not.
According to Marx and Stern [13], most COFs require an incisional biopsy, because their presentation suggests more aggressive disease. After a diagnosis is established, a panoramic radiograph is sufficient for treatment planning.
Mohanty et al. [14] reported on the rare occurrence of unilocular intrabony pathology in the mandibular anterior teeth, similar to our case. Of 17 cases found in a 10-year review of records, nine different pathologies were seen, although clinical and radiographic signs and symptoms were similar: ameloblastoma, adenomatoid odontogenic tumor, odontogenic keratocyst, two ossifying fibroma, idiopathic bone cavity, dentigerous cyst, radicular cyst, central giant cell granuloma, and calcifying odontogenic cyst. Careful histologic examination would therefore appear to be essential to a correct diagnosis.
Sheikhi et al. [15] observed a lesion in a 37-year-old patient’s maxilla, and a range of clinical and radiographic (intraoral, panoramic, cone beam computed tomography) did not provide a diagnosis. The differential diagnosis included chondrosarcoma or osteosarcoma, fibrous dysplasia, odontogenic cyst, squamous cell carcinoma, calcifying odontogenic cyst (Gorlin cyst), and calcifying epithelial odontogenic tumor (Pindborg tumor). The well-defined border of what they termed a “central cementifying fibroma” helped differentiate it from the aggressive sarcomas and carcinomas.
Scholl et al. [16] reviewed cysts and cystic lesions of the mandible. They noted that many nonodontogenic lesions can mimic odontogenic lesions, including benign fibro-osseous lesions (conventional or juvenile ossifying fibroma, focal or periapical cemento-osseous dysplasia, florid osseous dysplasia), traumatic bone cyst, lingual salivary gland inclusion defect, central giant cell granuloma, brown tumor of hyperparathyroidism, arteriovenous malformation, and mucoepidermoid carcinoma. Like Mohanty et al. [14], Scholl et al. concluded that “microscopic tissue evaluation is generally necessary to accurately identify the lesion”.
TREATMENT AND RECURRENCE
The treatment of COF involves conservative surgery through enucleation of the lesion and the use of a curette to heal the remaining cavity. These lesions readily separate from their bony crypt and show no evidence of bony infiltration. The resultant bony cavity is closed at the mucosal level without the need for drains or packing [13].
In spite of the frequent ease of removal of COF, because it does not adhere to bone or tooth structures, uncommon recurrences have been attributed to insufficient curettage. Due to its benign, slow growth, a clinical identification of recurrent or residual disease might not be identified until several years later. Dunlap and Barker [17] presented two cases of maxillary COF treated by curettage with follow-up of 9 years and 10 years, respectively, with no evidence of recurrence. However, some recurrent cases have been reported. Heimdal et al. [18] in 1980 reported a recurrence 9 years after surgical removal. In 1986 Svirsky et al. [5] reported a 13% (2 out of 15 cases) rate of recurrence. Jones et al. [19] reported a case that recurred 16 months after surgery. According to Marx and Stern [13], if a recurrence is observed, the original pathological specimen, as well as a biopsy specimen, should be reviewed. Despite the low recurrence rate, postoperative monitoring should be carried out for at least 5 years after surgical removal.
CONCLUSION
Although COF is a rare lesion of the periodontium, general dentists and periodontists should consider the presence of COF because this lesion closely resembles endodontic lesions. COF lesions are often diagnosed in the second and third decades of life [20].
A well-circumscribed, low-density, radiolucent lesion presented between the two mandibular central incisors. The buccal and lingual cortical plates appeared to be thinned and expanded (Fig. 1f).
Potential radiolucent lesions may include lateral periodontal cyst, central giant cell granuloma, ameloblastoma, or other odontogenic cysts. Therefore, biopsy was suggested to more precisely define the biological nature of the lesion. Surgical incisional biopsy was performed to diagnose the lesion, which was firm but asymptomatic and created a large diastema, widened the periodontal ligament, and led to divergent tooth roots over a 1-year period. Histological finding was COF, WHO type. The tumor was successfully removed, and periodontal regenerative therapy was performed to rebuild the hard and soft tissue that had been compromised as a result of tumor expansion. Preventive measures were taken before the tumor was able to spread further, which may have led to severe bone loss and possible complications. The site was grafted, with excellent results. The patient was satisfied with the outcome of treatment.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
ACKNOWLEDGMENTS
The assistance of Jennifer P. Ballinger, ELS, in finalizing the manuscript is gratefully acknowledged.
ABOUT THE AUTHORS
Ahmad Soolari is a Diplomate of American Board of Periodontology. He has a specialty Certificate in Peridontics and MS degree from the University of Rochester, Rochester New York. Dr. Soolari operates a specialty practice in the Silver Spring, Gaithersburg and Potomac area of Montgomery County, Maryland, USA. He can be reached at asoolari@gmail.com.
Asghar Khan has BDS degree. He is currently taking courses to prepare for dental school in the USA. He became a Certified Dental Radiation Technologist (CDRT) while working in a periodontal office. He can be reached by email at azukhan0587@gmail.com.


