All published articles of this journal are available on ScienceDirect.
Use of Ultrasonic Bone Surgery (Piezosurgery) to Surgically Treat Bisphosphonate-Related Osteonecrosis of the Jaws (BRONJ). A Case Series Report with at Least 1 Year of Follow-Up
Abstract
This preliminary work documents the use of a powerful piezosurgery device to treat biphosphonate-related osteonecrosis of the jaw (BRONJ) in combination with classical medication therapy. Eight patients presenting 9 BRONJ sites were treated, 2 in the maxilla and 7 in the mandible. Reason for biphosphonate (BiP) intake was treatment of an oncologic disease for 5 patients and osteoporosis for 3. The oncologic and osteoporosis patients were diagnosed with BRONJ after 35-110 months and 80-183 months of BiP treatment, respectively. BRONJ 2 and 3 was found in 4 patients. Resection of the bone sequestrae was performed with a high power ultrasonic (piezo) surgery and antibiotics were administrated for 2 weeks. Soft tissue healing was incomplete at the 2-week control but it was achieved within 1 month. At the 1-year control, soft tissue healing was maintained at all patients, without symptom recurrence. One patient with paraesthesia had abated; of the 2 pa-tients with trismus, one was healed, severity of the second trismus abated. This case report series suggests that bone resection performed with a high power ultrasonic surgery device combined with antibiotics might lead to BRONJ healing. More patients are warranted to confirm the present findings and assess this treatment approach.
INTRODUCTION
Biphosphonates (BiPs) have been a treatment of choice for a variety of diseases. It includes osteoporosis, skeletal-related events associated with bone metastases of solid tumors such as cancer of breast, prostate and lung, and management of lytic lesions in the setting of multiple myeloma [1-5]. Although BiPs do not improve cancer-specific survival, they significantly affect the life quality of patients suffering from advanced cancer involving the skeleton. Similarly, the clinical efficacy of oral BiPs for the treatment of osteopenia/osteoporosis has been well established. Up to 2007, over 225 million oral BiPs prescriptions have been dispensed worldwide [6].
According to the type of disease, BiPs are administrated through various ways, oral, parenteral or intra-venous. Oral intake is preferred to overcome osteoporosis, while intra-venous intake is given to tumoral patients [3, 7].
Osteonecrosis of the jaws (ONJ) have been recently linked to BiP treatment. Frequency of BRONJ has been found to vary between 1/10,000 to 1/100,000. Patients receiving oral bisphosphonate therapy are at a considerably lower risk of BRONJ than cancer patients treated with monthly IV bisphosphonates [8].
Oral and maxillofacial surgeons were the first to report non-healing cases of exposed bone in the maxillofacial region in patients treated with IV bisphosphonates [9, 10]. Generally, radiographic examination connected to soft tissue infection reveals images of bone necrosis with evidence of bone sequestrate [11].
Attention is usually drawn toward BRONJ when the 3 following characteristics are met [9]:
- bone has been exposed to the oral cavity despite treatment for more than 8 weeks,
- patient is treated or has been treated with BiPs,
- patient has no history of radiation therapy to the jaws.
The classification of BRONJ according to stages has been recently revised by Ruggiero et al [9]. It is now the following:
Stage 0: No clinical evidence of necrotic bone, however nonspecific clinical findings and symptoms are present,
Stage 1: Exposed and necrotic bone in asymptomatic patients without evidence of infection,
Stage 2: Exposed and necrotic bone associated with infection as evidenced by pain and erythema in region of exposed bone with or without purulent drainage,
Stage 3: Exposed and necrotic bone in patients with pain, infection, and one or more of the following: exposed and necrotic bone extending beyond the region of alveolar bone, (i.e. inferior border and ramus in the mandible, maxillary sinus and zygoma in the maxilla) resulting in pathologic fracture, extraoral fistula, oral antral/oral nasal communication, or osteolysis extending to the inferior border of the mandible or the sinus floor.
When BRONJ class 1 is confirmed, the treatment consists of symptomatic treatment with oral antibiotics, oral antibacterial mouth rinse with chlorhexidine, pain control and superficial debridement to relieve soft tissue irritation. When BRONJ classes 2 or 3 are assessed, the same as above is tried. When it fails, the treatment is more aggressive; it involves surgical debridement/resection for longer term palliation of infection and pain [9]. In all cases, soft tissue healing is not easily achieved after surgery.
Several methods have been implemented to try to regulate soft tissue and bone healing, they are medication [11, 12], surgical resection [13-17], hyperbaric oxygen therapy [18], laser biostimulation [13, 14], autologous platelet-rich plasma [19]; but their efficiency could not be proven [9, 20]. Surgeons often feel powerless with this severe disease [17, 20].
The aim of the present case report series is to document the application of ultrasonic surgery as a new surgical tool to treat BRONJ patients that did not respond to medical treatment alone. A follow-up of at least 1 year was introduced in order to check for recurrent symptoms.
MATERIALS AND METHODOLOGY
Patient Enrollment in the Present Case Series
Inclusion criteria in the present case series were: (i) patients were diagnosed with BRONJ at various stages of clinical progression (Table 1), (ii) after they did not respond to classical medical treatment, patients were planned for surgical treatment. Surgery was performed by using vibrating tips connected to a high power ultrasonic (piezo) device, (iii) patients had to be followed for at least 12 months after surgery in order to be able to check for recurrent symptoms. This excluded patients that died earlier because of their primary oncologic disease or were prevented to attend because of their general health situation.
Detail of the Treated Patients and Related Information
| Nb | Pat | Sex | Age | Primary disease | BiP Treatment | Length of BiP treatment (months) | Triggering cause of BRONJ | Osteonecrotic area | ONJ Classification | Time between start of BiP intake and BRONJ diagnosis (months) | Time between end of BiP intake and BRONJ diagnosis (months) | Time between surgery and last follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | MA | F | 79 | Breast cancer | Zoledronate (Zometa) | 15 | Extraction of # 43, 44 | Mandible, Left anterior area | ONJ 3 | 39 | 0.5 | 27, deceased |
| 2 | IG | F | 77 | Osteoporosis | Alendronate (Fosavance) | 35 | Extraction of # 46 | Mandible, Posterior at # 46 | ONJ 2/3 | 88 | 0 | 38 |
| 3 | DF | M | 68 | Multiple myeloma | Zoledronate (Zometa) | 38 | Extraction of # 36,37, 46,47 | Mandible, Bilat. posterior at # 36,37 & 46,47 | ONJ 3 | 110 | 6 | 32 |
| 4 | BA | M | 77 | Prostate cancer | Zoledronate (Zometa) | 33 | Traumatic ill-fitted prosthesis | Mandible, Anterior at # 33,34 | ONJ 2 | 90 | 3 | 26 |
| 5 | SM | F | 81 | Osteoporosis | Alendronate (Fosavance) | 32 | Extraction of # 11,12 | Maxilla, Anterior at # 11,12 | ONJ 2 | 80 | 0 | 23 |
| 6 | GP | M | 60 | Multiple myelome | Zoledronate (Zometa) | 20 | Extraction of # 16,17 | Maxilla, Posterior at # 16,17 | ONJ 3 | 53 | 1 | 15 |
| 7 | NL | F | 69 | Osteoporosis | Alendronate (Alendros 70) | 70 | Extraction of # 47 | Mandible, Posterior at # 47 | ONJ 2 | 183 | 3 | 14 |
| 8 | MS | F | 59 | Multiple myelome | Zoledronate (Zometa) | 13 | Extraction of # 43, 44 | Mandible, Left anterior area | ONJ 2 | 35 | 1 | 13 |
From January 2008, eight consecutive patients entering the inclusion criteria were treated in Gallipoli Hospital (Gallipoli, Puglia, Italy) for BRONJ by a single oral surgeon (GG); they were 5 women and 3 men, mean age was 71.3 ± 8.6 (range 59-81). Six mandibles were diagnosed with osteonecrosis, 3 in the anterior region and 3 in the posterior; in the maxilla, one was in the anterior region and one in the posterior. One mandible had bilateral necrosis in the posterior area; overall 9 sites were treated. Dental events having triggered osteonecrosis were tooth extraction in 7 patients and an ill-fitting prosthesis in 1 of them (Table 1).
Patients have been under BiP treatment for different reasons: 5 to treat various neoplasic diseases associated with metastatic bone lesions and 3 for osteoporosis (Table 1). Five patients received Zoledronate (Zometa, Novartis Farma, Orrigio, Italy) and 3 received Alendronate; 2 as Fosavance (Merck, Sharp & Dohme, Rome, Italy) and 1 as Alendros 70 (Abiogen Pharma SpA, Pisa, Italy). All tumoral patients (Table 1) were treated with chemotherapy, immuno-depression or hormonal therapy. BiP intake lasted for an average of 32.0 ± 18.1 months (range 15-38 months). Osteonecrosis was diagnosed 84.5 ± 47.6 months in average (range 35-110 months) after initiation of BiP intake and 2.8± 2.4 months in average (range 0-6 months) following the end of the BiP treatment. Two patients were diagnosed with osteonecrosis during the BiP treatment; intake was subsequently interrupted (Table 1).
Treatment Protocol
When osteonecrosis was diagnosed (Table 1), conservative Chlorhexidine 0,2% rinsing was recommended two times per day and an antibiotic therapy cycle of 2 x 2 weeks separated by 1 week was administrated; it consisted of amoxicillin and Clavulanic acid (2g/d) and Metronidazole (1g/d) [20]. If gingival healing was not achieved, surgical treatment was considered.
Symptoms and Clinical Signs
Symptoms and clinical signs were recorded for each patient before surgery and thereafter at the various milestones (Table 2). Patients treated for osteoporosis showed osteonecrosis superposed with infection, recalling osteomyelitis. With the oncology patients, infection appeared as the result of exposure of bone. Trismus was found at 2 patients, one of them was slight; another patient suffered from paraesthesia (Table 2).
Piezosurgery Device
The surgical treatment was performed with the UBS device (Resista, Omegna, Italy), a powerful piezosurgery device with titanium alloy tips [21, 22] (Fig. 1a, 1b) and a peak power of 90 W [22].

Ultrasonic surgery device and the used tips.
a. Ultrasonic device with the vibrating handpiece. Peak power is 90 W at level 9; at level 5 out of 9, power is 72 W.
b. The vibrating tips that have been used to remove the necrotic bone. From above, the bone scraper tip; the straight saw to cut on the vestibular and lingual/palatal sites; the orthogonal saw to cut on the mesial and distal sites of the sequestrum; the angled round tip to smoothen the occlusal sites of the alveolar ridge after removal of the sequestrum; the long straight round tip for an easier access of the posterior area on the vestibular and lingual sides. All tips are made of titanium aluminum vanadium alloy (Ti gr 5).
Activation of the ultrasonic device leads to cavitation and the latter may have a killing effect on bacteria in suspension [23, 24]. It has been attributed to membrane rupture following numerous cycles of powerful bubble explosions at the vicinity of the living germs.
Three vibrating types of tips (Fig. 1b) were implemented with the power set at 72 W (level 5 out of 9) [24]. The bone scrapper tip served to remove the superficial necrotic bone. The 2 saws, a straight and an orthogonal one, were used to separate the bone sequestrae. The 2 round tips, a short for the anterior area and a long straight one to easily access the posterior vestibular and lingual sectors were convenient to smooth down the sites before soft tissue closure.
Medical Protocol
Antibiobiotic therapy (amoxicillin and Clavulanic acid 2g/d and Metronidazole 1g/d) was implemented for 2 weeks, starting 1 day before ultrasonic surgery.
Ultrasonic Surgery Protocol
Following a loco-regional anesthesia with Mepivacaine chlorhydrate without vasoconstrictors (Optocain, Molteni dental, Scandicci, Italy), a flap was raised and the soft tissues were reclined to provide full access to the bone. Bone was resected with the various vibrating tips.
Follow-up and Success Criteria
After surgery, patients were controlled 2 weeks after surgery, at 1 month, 6 months, 1 year and then for the need of the present report; patients could also attend in case they needed. Symptoms and clinical signs were assessed at each visit; orthopantomograms were taken to get radiological information at 6 months and later.
Clinical success criteria were: (i) symptom abatement or complete resolution, (ii) soft tissue coverage of the exposed bone, (iii) healthy soft tissue at the treated sites. X-ray examination was not part of the assessment because the indication of healing cannot be proven radiologically [16].
RESULTS
During surgery, local bone finding was different according to the reason for BiP treatment, osteoporosis or neoplasm. With the osteoporotic patients, necrotic bone was not as extensive as for the oncology patients; it was rather disseminated among vital bone. In this case, the bone scraper tip and the round one were used to remove the necrotic non-bleeding areas. At the cancer patients, the necrotic bone was scraped and the bone sequestrae were then sectioned with the straight and the orthogonal saws. Bone was further removed with the bone scraper and the round tips until bleeding was assessed all over the remaining bone. Overall, the local tissues were exposed to several minutes of powerful cavitation. The granulation tissue was removed and a gingivoplasty was performed before suturing the gingiva. In all cases, primary intention closure was obtained.
Histological sampling confirmed osteonecrosis and infiltration of lymphoplasma cells; 2 patients suffered from mycosis in addition of osteonecrosis.
Figs. (2-4) are showing some of the treated cases. Table 2 provides the symptoms recorded for each patient and their progress following surgery. At the 2-week control, healing was delayed, still incomplete but ongoing. At the 1-month control, all symptoms either abated or disappeared (Table 2). The gingiva was healthy and covered the treated area; a slight invagination was observed, probably due to a loss of bone support. In one patient out of 2, the recorded trismus abated but persisted. In the patient that suffered from paraesthesia the severity was reduced. At the 6-month and 1-year controls, the gingivae were healthy without recurrent symptoms. Average follow-up was 23.0 months (min 12 m, max 38 m), one patient deceased after the last control at 27 months.
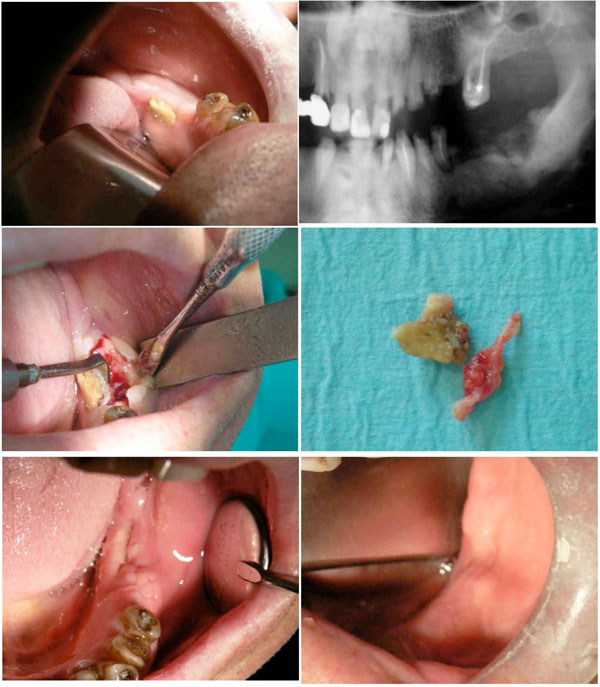
Treatment of a BRONJ case in the mandible.
a. Preoperative clinical situation. ONJ 3 has been diagnosed after 110 months of Zoledronate intake to treat multiple myeloma. Tooth extraction was considered as the triggering event.
b. Radiographic examination. Note the large necrotic area in the posterior region.
c. Section of the sequestrum with the vibrating tips of the piezosurgery device.
d. Bone sequestrum and soft tissue removed by the vibrating tips.
e. Healing at 1 month. The soft tissues are showing invagination.
f. One-year control. Healing of the necrotized and infected area is complete.
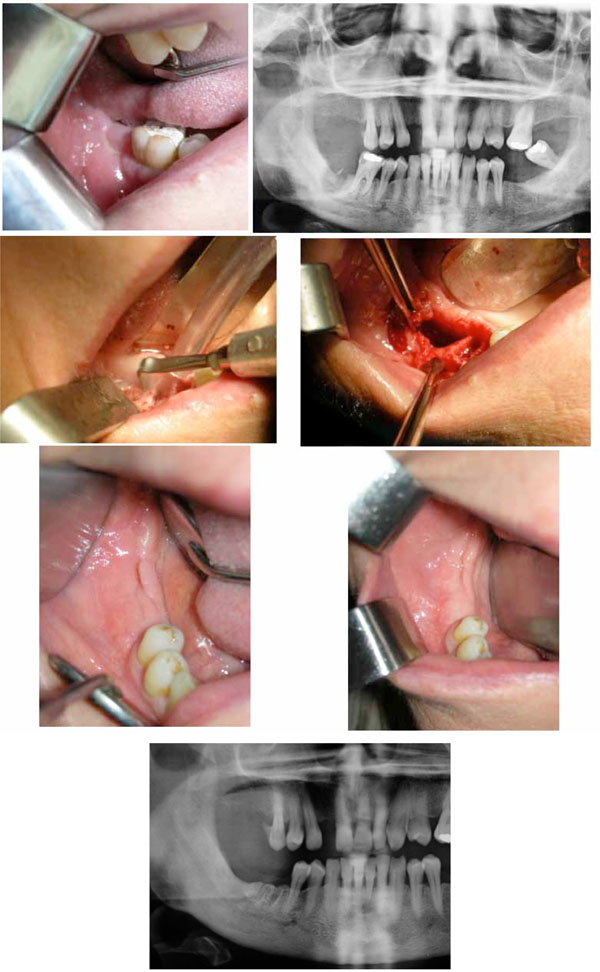
Treatment of a BRONJ case in the mandible.
a. Preoperative clinical situation. ONJ 3 has been diagnosed after 72 months of Alendronate treatment for osteoporosis. Tooth extraction was considered as the triggering event.
b. Preoperative radiograph. Note the area of necrotic bone at the right second molar site in the mandible.
c. Surgical section of the sequestrum with a vibrating tip of the ultrasonic surgery device.
d. Surgical site before soft tissue closing. Note the large defect and the site of the extracted first molar.
e. Healing at 1 month. The soft tissues are showing invagination.
f. One-year control. Note the complete healing of the site.
g. Radiograph at the 1-year control. Note the satisfactory radiologic aspect at the necrotized site.
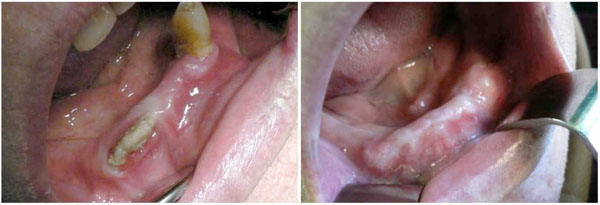
Treatment of a BRONJ case in the mandible.
a. Preoperative clinical situation. ONJ 2 has been diagnosed after 90 months of Zoledronate treatment for prostate cancer. Tooth extraction was considered as the triggering event.
b. Clinical situation at the 1-year control. Note the clinical aspect of the gingiva.
Clinical Symptoms Noted at each Patient and Status at the 1-Year Control. A Slight Trismus was Still Noted at One Patient and the Paresthesia Noted in a Single Patient Abated Significantly. Note that the Osteoporosis Patients (pat 2, 5, 7) did not have Exposed Bone
| Pat 1 | Pat 2 | Pat 3.1 | Pat 3.2 | Pat 4 | Pat 5 | Pat 6 | Pat 7 | Pat 8 | |
|---|---|---|---|---|---|---|---|---|---|
| Spontaneous bleeding | healed | healed | healed | healed | healed | healed | healed | healed | healed |
| Purulent exudate | healed | healed | healed | healed | healed | healed | healed | healed | healed |
| Intense localized pain | healed | healed | healed | healed | healed | healed | healed | healed | healed |
| Feeding difficulty | healed | healed | healed | healed | healed | healed | - | healed | healed |
| Halitosis | healed | healed | healed | healed | healed | healed | healed | healed | healed |
| Tumefaction | healed | healed | healed | healed | healed | healed | - | healed | - |
| Exposed bone | healed | - | healed | healed | healed | - | healed | - | healed |
| Soft tissue ulceration | healed | healed | healed | healed | healed | healed | - | healed | - |
| local swelling | healed | healed | healed | healed | healed | healed | - | healed | - |
| Fistula | - | healed | - | - | - | healed | - | healed | - |
| Trismus | - | abated | - | - | - | - | - | healed | - |
| Paraesthesia | - | - | - | - | - | - | - | abated | - |
DISCUSSION
In the hospital environment where patients were treated, the cancer patients receiving BiPs have had a high mortality, shortly after treatment of the BRONJ symptoms. It is therefore rather difficult to evaluate the success of the management over time. This is the reason why this case series focused only on patients that could be followed for at least 1 year.
As previously reported by others [25, 26], patients that received BiPs for oncologic reasons had more advanced necrosis than patients treated for osteoporotic reasons.
Several methods have been tried to boost the healing of the soft and hard tissues involved in the BRONJ symptoms [12-19]. Usually, they are aiming at eliminating pain and the other clinical symptoms, control the local infection, minimize the progression of bone necrosis and avoid repetition of necrosis.
For the first time, ultrasonic bone surgery was applied to the BRONJ patient in need of surgical treatment. The assumption of a helpful application toward abating or healing the infectious aspect of the syndrome was drawn from the
Preoperative clinical situation. ONJ 3 has been diagnosed after 110 months of Zoledronate intake to treat multiple myeloma. Tooth extraction was considered as the triggering event.
Radiographic examination. Note the large necrotic area in the posterior region.
Section of the sequestrum with the vibrating tips of the piezosurgery device.
Bone sequestrum and soft tissue removed by the vibrating tips.
Healing at 1 month. The soft tissues are showing invagination.
One-year control. Healing of the necrotized and infected area is complete.
Preoperative clinical situation. ONJ 3 has been diagnosed after 72 months of Alendronate treatment for osteoporosis. Tooth extraction was considered as the triggering event.
Preoperative radiograph. Note the area of necrotic bone at the right second molar site in the mandible.
Surgical section of the sequestrum with a vibrating tip of the ultrasonic surgery device.
Surgical site before soft tissue closing. Note the large defect and the site of the extracted first molar.
Healing at 1 month. The soft tissues are showing invagination.
One-year control. Note the complete healing of the site.
Radiograph at the 1-year control. Note the satisfactory radiologic aspect at the necrotized site.
Preoperative clinical situation. ONJ 2 has been diagnosed after 90 months of Zoledronate treatment for prostate cancer. Tooth extraction was considered as the triggering event.
Clinical situation at the 1-year control. Note the clinical aspect of the gingiva. bacteriocidal effect we previously observed in vitro on Gram positive (Escherichia coli) and Gram negative bacteria (B. subtilis) [24]. It came also from a previous positive result while treating the diabetic foot [27] and placing dental implants successfully in chronic and acute infected post-extraction sites after local ultrasonication [28].
An additional element was the findings of Preti et al. [29] while investigating the early stages of bone healing. These authors showed that more inflammatory cells were present in the bone samples prepared by drilling when compared to piezosurgery. Furthermore, the local increase in BMP-4 was earlier when the vibrating tips were applied [29].
In fact, the treatment that was applied in these patients is a combination of a medical protocol with antibiotics and a surgical one with a powerful piezosurgery device. The infected and necrotic tissues underwent a powerful cavitation effect during several minutes.
Some authors reported that microbial biofilms can be involved in the BRONJ process [30, 31]. Thus, the affected bone surface might offer an optimal microbial support, due to the coating process that develops between BiPs and hydroxyapatite [32]. Microbial aggregation might increase the inflammatory and necrosis status of the tissues [30-32].
In this context, application of ultra-sonic vibration and its related powerful cavitation might have decreased the microbial critical mass around the involved bone and allowed synergy with the medical treatment. This would be in line with the report that showed low-frequency surface acoustic waves being able to eradicate biofilm-residing bacteria by > 85% when applied simultaneously with an antibiotic in three clinically relevant species: Escherichia coli, Staphylococcus epidermidis and Pseudomonas aeruginosa [33].
In these patients, tooth extraction had a triggering effect on bone necrosis. The above arguments related to biofilm disruption could suggest that performing extraction with the vibrating tips might have avoided or at least abated such triggering effect. For this purpose, it might also be appropriate to add 2 minutes of ultrasonication of the socket to carefully clean all its walls. But all that needs further confirmation.
In this case series, healing was incomplete after 2 weeks but still progressing toward closure of the gingiva. At the 1-month control, complete closure of the surgical wounds was observed in addition to the disappearance of swelling and the other symptoms described in Table 2. A slight trismus persisted up to the 1 year control. The reason for these successful results is not fully understood; but the assumptions that led to apply ultrasonic surgery to treat this type of pathology might have been relevant.
CONCLUSION
In this work and for the first time, ultrasonic bone surgery was introduced as the surgical tool to successfully treat patients with BRONJ that needed surgical treatment. More patients are warranted to undergo the present combined medical and surgical protocol before drawing any conclusion. But the data should encourage further in vivo/in vitro experimental protocols about the involvement of ultrasonic surgery into the treatment of BRONJ.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
ACKNOWLEDGEMENTS
The authors want to thank doctorando Fourouzan Shapourovna Sebanoff for her active assistance during the conception of the paper


