All published articles of this journal are available on ScienceDirect.
Salivary Alpha-Amylase Activity and Salivary Flow Rate in Young Adults
Abstract
The secretion of salivary alpha-amylase (sAA) is more associated with psychoneuroendocrinological response to stress than with the flow rate and age. The aim of this cross sectional study is to build an explanatory model based on patterns of relationship between age 20-39 in resting and stimulated saliva under no stressful condition in healthy volunteers. Both resting and stimulated saliva were collected from 40 subjects. The sAA values were log-transformed, the normality assumption was verified with the Shapiro-Wilk test and the reliability of the measurements was estimated by the Pearsons’ r correlation coefficient. The estimated model was based on the theory of the Linear Mixed Models. Significant mean changes were observed in flow rate and sAA activity between resting and stimulated saliva. The final model consists of two components, the first revealed a positive correlation between age and sAA while the second one revealed a negative correlation between the interaction of age × flow rate in its condition (resting or stimulated saliva), with sAA. Both flow rate and age influence sAA activity.
INTRODUCTION
Salivary α-Amylase (sAA) is one of the most plentiful components in saliva, accounting for 10–20% of the total protein content [1]. sAA is locally produced by the highly differentiated epithelial acinar cells of the exocrine salivary glands, mostly of the parotid glands [2]. sAA contributes in food digestion through the hydrolysis of starch to glucose and maltose [3]. Additionaly, sAA has been suggested to prevent bacterial attachment to oral surfaces and to enable bacterial clearance from the mouth [4].
Recent observations indicate a relation between sAA secretion and experience stressful condition. The enzyme concentration increases under both physical stress, such as treadmill exercise, running, bicycle exercise and cold exposure [5-9] and psychological stress as well such as watching highly negative emotional pictures of mutilation or accidents, participating in collegiate level individually oriented athletic competition, written examination, and Trier Social Stress Test (TSST) [10-15]. When the concentration of catecholamines (epinephrine and nor-epinephrine) increases in the blood due to stress, the sAA concentration also increases [16].
Salivary flow rate may be affected from several factors: apart from perceived stress [4] and depression [17] other variables are age [18], alcohol consumption [19], exercise intensity [20], as well as cancer and radiation treatment [21]. In all these studies, the fluctuation of the salivary flow rate coincides with the fluctuation in the sAA concentration. However, it is unclear whether the fluctuation of sAA concentration is an effect of the altered salivary flow rate or if it directly depends on the above mentioned stress factors. One study indicates that flow rate is not a confounder of stress-induced sAA activation [22]. Nevertheless, the method used for the determination of the salivary flow rate lacks relevant control. Contrary to other researchers Mackie & Pangborn (1990) concluded that mastication increases the salivary secretion rate but not the concentration of protein and sAA. They failed to prove a relationship between sAA concentration and salivary flow rate, although they found indications for mastication induced changes in salivary protein concentration [23]. The importance of the sampling techniques, duration times, and locations on sAA determination and the relationship between salivary flow rate and sAA activity, as well, have been pointed out by other authors too [24].
Thus, it remains unclear whether an increase of the salivary flow rate alters the sAA concentration in the absence of a systemically affecting factor. The aim of this cross sectional study was to examine the sAA activity in resting and stimulated saliva sampled from young adults under no stressful condition. An explanatory model is suggested based on patterns of relationship between age, flow rate, and sAA activity in resting and stimulating saliva.
MATERIALS AND METHODOLOGY
Saliva Samples
Both resting and stimulated saliva was collected from a total of 40 healthy volunteers, ranged in age from 20 to 39 years (mean age 25.21±5.06 years). All subjects were undergraduates, postgraduates, or PhD dental students and declared medical health. The subjects were informed and signed a written informed consent. The study protocol was approved by the Ethics committee of the Dental School.
Sampling sessions were limited to the hours between 10:00 and 11:00 AM to minimize the effect of diurnal variations [25]. The subjects were instructed to refrain from exercising [9], eating [26], smoking [27], drinking any beverages [28] except water 1 h before saliva sampling. Initially, resting whole saliva was collected. The volunteers were supervised and instructed to be comfortably seated with their head tilted slightly forward and to avoid making orofacial movements. Additionally, they were instructed to swallow any saliva in their mouth, immediately before the collection started. In brief, saliva was allowed to accumulate in the floor of the mouth and to drool into the test tube for 15 minutes [29]. After a 10-minute pause, a consecutive 3-min sample of stimulated saliva was collected. The stimulus was both gustatory and masticatory provided by chewing a flavoured standard sized sugar free gum (ELMA® Chios Greece). The volunteers were asked to chew the gum for 2-min and to swallow the saliva produced. Thereafter, they continued chewing the gum for 3 minutes and spit the saliva volume secreted into a test tube. The rate of recurrence of stimulation was approximately 70 chews/min as suggested [29]. The volume of each saliva sample was measured to the tenth of ml. Aliquots of the samples were centrifuged to remove any particulated material and the supernatants were stored at -80°C, until analysed [30].
Determination of sAA Activity
The sAA activity of the samples was detected through the enzymatic hydrolysis of the chromogenic substrate 2-chloro-4-nitrophenyl α-D maltotrioside [31]. In brief, the reaction mixture contained 5 mM chromogenic substrate, 0.03% bovine serum albumin, 5mM CaCl2, 50mM KSCN, and 0.03% NaN3 in 50 mM Morpholinoethansulfonic acid (MES) buffer, pH 6.0. The reaction was followed spectrophotometrically at 405 nm.
Statistical Analysis
From the 40 volunteers following the inclusion criteria 2 were excluded from the analysis since their sAA activity values were 3 SD below the mean value (in at least one of the two conditions stimulated or resting saliva). The values were log-transformed, and the normality assumption was checked with the QQ-plot and Shapiro-Wilk test [32]. The reliability concerning measurements on resting and stimulating values of log(flow rate) and log(sAA) were evaluated with Pearson Correlation coefficient (N>15). Differences between stimulated and resting saliva log(flow rate) were statistically analyzed with a linear mixed model. Then the log(flow rate) and log(sAA) differences between stimulated and resting saliva were computed and the two new variables were afterwards used in two cluster analysis with the k-means method [33]. Each cluster analysis revealed two cohesive groups of individuals (four groups in overall) and for each one of these groups the correlation between log(age), differences on log(flow rate) and differences on log(sAA) was estimated with Pearson’s r correlation coefficient. The results showed some moderate linear relationship between the above mentioned variables. Based on these linear relationships among pairs of groups a series of linear mixed models as conducted with log(sAA)-mean[log(sAA)] as the dependent variable, condition (resting or stimulated saliva flow) as a fixed inter subject factor, subjects as random factor, and log(age)-mean[log(age)] and log(flow rate)-mean [log(flow rate)] as covariates. Each covariate was preferred to be centered at the mean in order to lead to a clearer interpretation of the estimated coefficients [34]. The null hypothesis concerning the significance of the random factor, and the null hypotheses concerning the choice of the best-fitted model were tested with the difference of the -2REML (Restricted Estimated Maximum Likelihood) log-likelihood estimation and Chi-Square test (in the first case the estimation was done with REML while in the second case ML estimation was [34]. The normality assumption for the residuals and the scatter plot of predicted values vs. residuals were used to check the validity of the best fitted model. The analysis was performed with SPSS 16.0 and the level of statistical significance was set at p<0.05.
Results
Descriptive statistics of the data set are shown in Table 1. A statistically significant increase in flow rate was observed, between resting and stimulated conditions (p<0.001, mixed linear model with condition as repeated effect and subject as a random factor). The reliability coefficients concerning measurements on resting and stimulated values of log(flow rate) and log(sAA) were r=0.51 and r=0.6 (p<0.001, Pearson’s correlation coefficient), respectively. Both coefficients are less than 0.7 [in order to compare groups, the intraclass correlation coefficient must be greater or equal to 0.7] [35] this proving the existence of intersubject heterogeneity. As suggested [32, 33] the delta scores (stimulated minus resting values) were calculated for log(flow rate) and log(sAA) activity and the resulted variables were denoted by Dlog(flow rate) and Dlog(sAA), correspondingly. Dlog(flow rate) and Dlog(sAA) were used subsequently in two cluster analyses with the k-means method [33]. Fig. (1) and Fig. (2) depict the scatterplots of Dlog(flow rate) vs. Dlog(sAA) according to the results of the two cluster analyses. Considering thegroups that emerged after the cluster analyses some moderate correlations were found between Dlog(flow rate), Dlog(sAA) and log(age). For subjects with decreased Dlog(sAA)<0.5, the correlation between log(age) and Dlog(flow rate) was r=0.51, p=0.042 (N=16) (Fig. 3), whereas for subjects with decreased Dlog(flow rate)<2.1, the correlation between Dlog(flow rate) and Dlog(sAA) was r=0.41, p=0.042 (N=25) (Fig. 4). Based on these findings, a linear mixed model was fitted to the data set, with the log(sAA) as the dependent variable, the condition (resting or stimulated) as the fixed within factor, the subject as random factor and the covariates log(age)-mean[log(age)] and log(flow rate)- mean[log(flow rate)] centered at the mean [34]. Results concerning the effect of the fixed factor the covariates and all their interactions (called fixed effects) under the full model are presented in Table 2. The likelihood ratio statistic of the significance of the random factor was calculated by subtracting the value of the -2REML log-likelihood of the full model without the random factor from the corresponding value of the full model containing the random factor, i.e. -99.391 - (-108.274) = 8.883. The p-value for the likelihood ratio statistic, was P(X2(1)>8.883) = 0.005, thus, revealing that the effect of the random factor (subject) is statistically significant and must be kept in the analysis. After the exclusion of the non-significant parameters from the full model (Table 2, see any line with p>0.05) a new linear mixed model was conducted (restricted model) and the results about the remaining fixed effects and their parameters are shown in Table 3 and Table 4, respectively. The likelihood ratio statistic for the comparison between the full and the restricted model was calculated by subtracting the value of the -2ML log-likelihood for the restricted model from the corresponding value of the full model, i.e. -92.244 - (-94.508) = 2.264. The p-value for the likelihood ratio statistic was P(X2(4)>2.264) = 0.687 indicating that the restricted model is better than the full model. The conditional residuals of the restricted model appear to follow a normal distribution [Kolmogorov-Smirnov test, p=0.200 and QQ-plot, (Fig. 5)]. The assumption of the constant variance for the residuals in the restricted model can be verified from the scatterplot of conditional predicted values vs. conditional residuals (Fig. 6). The chart shows no particular pattern and the values of the residuals seem to be symmetric around zero).
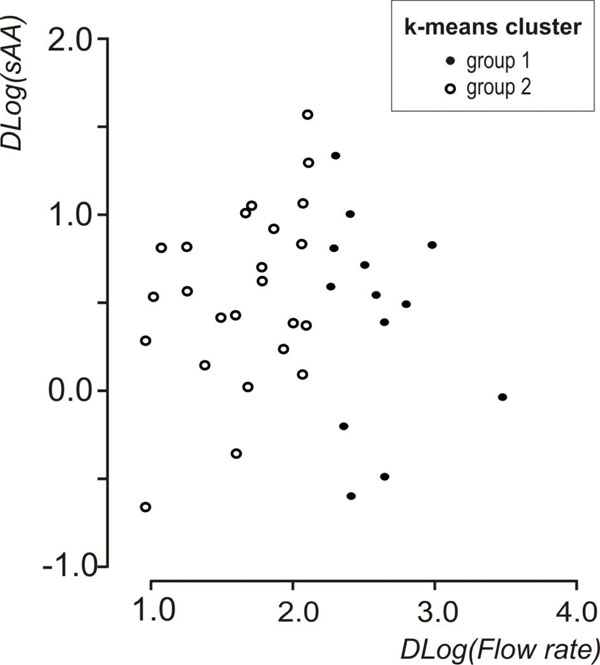
Scatterplot of Dlog(flow rate) vs Dlog(sAA) showing the groups that emerged after the k-means cluster analysis on Dlog(flow rate).
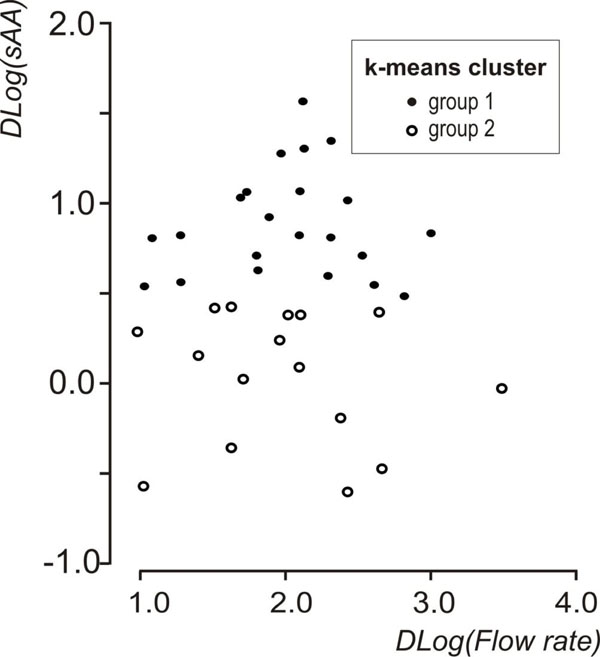
Scatterplot of Dlog(flow rate) vs Dlog(sAA) showing the groups that emerged after the k-means cluster analysis on Dlog(sAA).

Scatterplot of log(age) vs. Dlog(flow rate), showing subjects with decreased Dlog(sAA), r=0.51, N=16 (Dlog(sAA)<0.5).
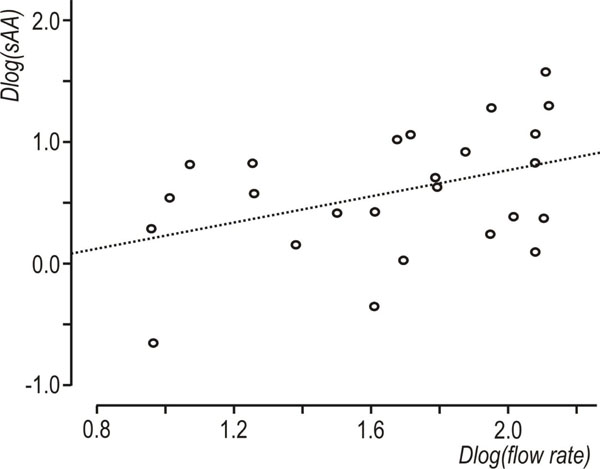
Scatterplot of Dlog(flow rate) vs Dlog(sAA), showing subjects with decreased Dlog(flow rate), r=0.41 N=25 (Dlog(flow rate)<2.1).
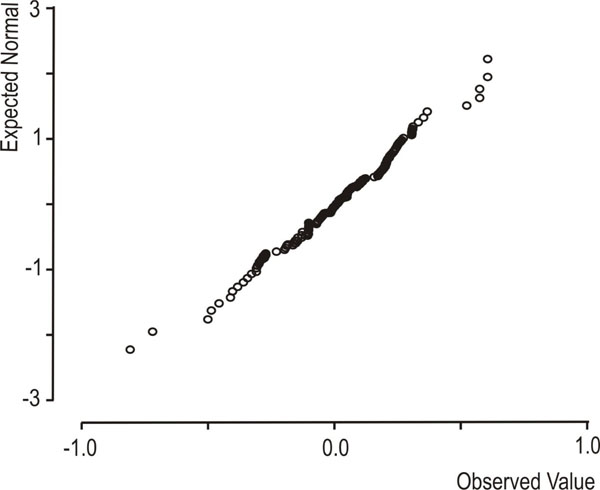
Normal QQ-plot of the conditional residuals from the restricted (best) model.
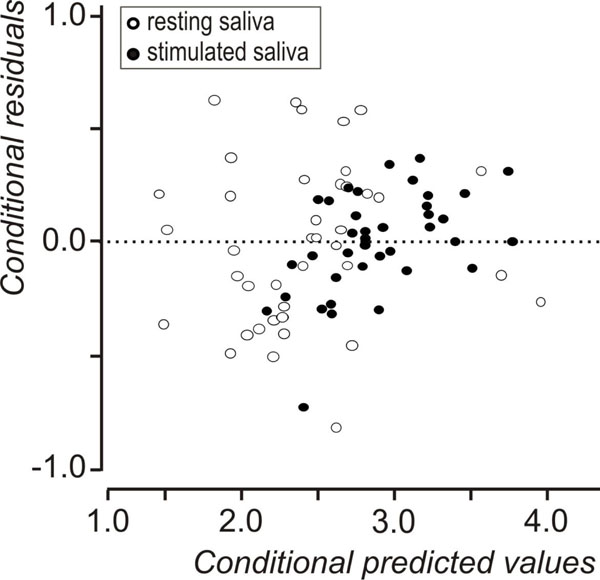
Conditional predicted values vs conditional residuals of the restricted (best) model.
Descriptive Statistics of the Data Set
| N | Minimum | Maximum | Median | Mean | Std. Deviation | |
|---|---|---|---|---|---|---|
| Age | 38 | 20 | 39 | 23.50 | 25.21 | 5.06 |
| flow rate (resting) ml/min | 38 | 0.06 | 1.06 | 0.33 | 0.35 | 0.23 |
| flow rate (stimulated) ml/min | 38 | 1.00 | 4.10 | 2.18 | 2.22 | 0.74 |
| sAA (resting) U/ml | 38 | 3.09 | 47.08 | 9.91 | 13.01 | 9.28 |
| sAA (stimulated) U/ml | 38 | 5.48 | 56.50 | 17.36 | 20.49 | 11.04 |
Results Concerning Hypothesis About Fixed Effects in the Full Linear Mixed Model, F(Numerator df, Denominator df) is the Corresponding Value of the F Distribution, with Corrected Degrees of Freedom
| Source | Numerator df | Denominator df | F | p-Value |
|---|---|---|---|---|
| Intercept | 1 | 36.580 | 1347.714 | <0.001 |
| log(age) – mean[log(age)] | 1 | 38.860 | 14.637 | <0.001 |
| Condition | 1 | 36.586 | 45.140 | <0.001 |
| Log(flow rate) – mean[Log(flow rate) | 1 | 57.711 | .000 | .984 |
| {Log(flow rate) – mean[Log(flow rate)}× log(age) – mean[log(age)] | 1 | 58.698 | 11.334 | 0.001 |
| condition × { Log(flow rate) – mean[Log(flow rate)} | 1 | 35.659 | .540 | .467 |
| condition × { log(age) – mean[log(age)]} | 1 | 39.575 | .014 | .908 |
| condition × {Log(flow rate) – mean[Log(flow rate)}× log(age) – mean[log(age)] | 1 | 35.844 | .997 | .325 |
Results Concerning Hypothesis about Fixed Effects in the Restricted Linear Mixed Model, F(numerator df, Denominator df) is the Corresponding Value of the F Distribution, with Corrected Degrees of Freedom
| Source | Numerator df | Denominator df | F | p-value |
|---|---|---|---|---|
| Intercept | 1 | 38.642 | 1539.503 | <0.001 |
| log(age) – mean[log(age)] | 1 | 38.962 | 17.753 | <0.001 |
| condition | 1 | 38.532 | 48.468 | <0.001 |
| {Log(flow rate) – mean[Log(flow rate)}× log(age) – mean[log(age)] | 1 | 55.228 | 12.899 | 0.001 |
Fixed Effects Parameter Estimates in the Restricted Model
| Parameter | Estimate | Std. Error | df | t | p-Value | 95% Confidence Interval | ||
|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||||
| Intercept | 2.942 | 0.074 | 40.592 | 39.997 | <0.001 | 2.794 | 3.091 | |
| b: log(age) – mean[log(age)] | 1.560 | 0.370 | 38.962 | 4.213 | <0.001 | 0.811 | 2.310 | |
| c: Condition | resting | -0.572 | 0.082 | 38.532 | -6.962 | <0.001 | -0.738 | -0.406 |
| stimulated | 0a | 0 | . | . | . | . | . | |
| fa: {Log(flow rate) – mean[Log(flow rate)}× {log(age) – mean[log(age)]} | -2.253 | 0.627 | 55.228 | -3.592 | 0.001 | -3.510 | -0.996 | |
a This parameter is set to zero because it is redundant.
Functional form of the proposed model sAA activity= e 2.942+1.56×b-0.572×c-2.253×fa
The results from the restricted model revealed:
- a statistically significant increase in log(sAA) at the stimulating condition (p<0.001, Table 4, coefficient for condition = rest),
- a positive association between sAA and age (p<0.001, Table 4, coefficient for {log(age) – mean [log(age)]}) and
- a negative association between sAA and the interaction of flow rate and age (p=0.001, Table 4, coefficient for {[Log(flow rate) – meanLog(flow rate)}× {log(age) – mean[log(age)]}).
DISCUSSION
As a surrogate non-invasive indicator of Sympathetic Nervous System (SNS) activation, sAA has widely been assessed the last two decades. An important concern is whether the salivary flow rate is a confounding factor that modifies sAA concentration. Saliva is a complex mixture derived from many different cell types within the glands innervated by several nerve types. Sympathetic stimulation decreases the salivary flow rate and increases the total protein salivary content [36] Increase of the salivary flow rate is due to parasympathetic nervous pathways stimulation, which is mainly responsible for regulating salivary flow rate [37] Our study was conducted under no stressful conditions, meaning that the parasympathetic stimulation mainly affected the flow rate and to a lesser extent the sAA secretion. The results revealed a 1.5 times mean increase of sAA in the stimulated saliva compared with the resting saliva, while the flow rate increased 6.3 times. Other studies measuring sympathetic stimulation (i.e. stressful conditions such as academic examination, skydiving, playing a stressful video game, watching a gruesome video, Trier Social Stress Test) showed sAA increase varied from 1.4-2.5 [4, 8, 22, 38-40]. Thus, the proportional increase of the sAA due to saliva flow increase under no stressful condition is of similar magnitude with the one recorded under stress.
sAA activity as a valid and reliable measure of SNS activity is not definite, as long as the influence of PNS on the sAA is not negligible. sAA concentrations can be influenced via synergistic sympathetic-parasympathetic interactions whereby parasympathetic activity amplifies sympathetic effects, and via parasympathetic activity through glands that are solely or mainly parasympathetically innervated, or through the effects of salivary flow rate (parasympathetically-mediated) [41]. Our results are in line with that showing the salivary flow rate as a confounder in sAA measurement. On the contrary Rohleder et al (2006) concluded that saliva flow rate have a minimal impact on sAA output [22]. This conclusion was mainly derived from experiments with small effects on flow rate like the one of the collection method. Similar findings were reported by others [38]. However, changes in salivary fluid secretion account for 25-40% changes in sAA activity [42], this implying the important role of salivary flow rate. A very recent study is in line with our findings strongly disputing the role of sAA as a marker of the SNS and suggesting that salivary chromogranin A but not sAA, correlates with cardiovascular parameters during high intensity exercise, confirming salivary chromogranin A as marker of psychological stress related to physical activity.
Based on the present findings a statistical explanatory model is suggested that defines the association between salivary flow rate, age, and sAA. The increase of sAA follows the increase of salivary flow rate under no stressful condition. This finding implies a triggering on the acinar cells of the salivary glands for higher sAA production. This is in line with a study searching the effect of oral stimulation on human parotid salivary flow rate and sAA secretion [43]. It is not well known whether the human parotid gland contains a sufficient store of sAA for continual secretion or whether sAA synthesis is stimulated and sufficiently rapid to replace the sAA being secreted. It was shown, though, that the parotid gland was able to maintain a high protein output for a long period of time [44]. Additionally resting saliva is mainly secreted by the submandibular glands and only about 20% derives from the parotid gland, which happens to be very rich in sAA [45,46]. Masticatory and gustatory stimulation drastically changes salivary protein composition due to the different responsivity of the parotid and submandibular glands to stimulation and the different amounts of sAA and other proteins that the secretion of these glands contains. Thus, in stimulated saliva the contribution of individual glands changes, whereby about half of all saliva is from the parotid glands. This condition highly affects sAA concentration in whole saliva as parotid saliva contains a 4-10-fold higher sAA amount than submandibular saliva [47]. Based on that it is recommended to specify the salivary flow rate for the sAA activity determination. Most of the studies measuring sympathetic stimulation through sAA activation [4, 8, 22, 38-40] disregard this parameter. Rohleder et al 2006 collected stimulated and unstimulated saliva under stressful condition (TSST). There was 2.7 times sAA increase in resting saliva and 1.8 in stimulated saliva. The synergistic effect of sympathetic, due to stress, and parasympathetic system, due to mastication on stimulated saliva reduced the sAA levels [22].
The statistical analysis of the present results revealed an influence of age on sAA activity. The relationship between flow rate and age changes through life span; in newborns the sAA activity is detected very low [48-50] and climbs to the adult levels within the first 3 years [51, 52]. Over the adulthood until older ages the sAA activity seems to remain unchanged [53, 54]. Studies have shown that with increasing age morphological changes of oral mucosa appear but only marginal alterations of salivary gland function and saliva composition are seen [55]. Nagler and Hershkovich (2005) reported a 62% lower resting saliva flow rate and higher sAA concentrations in elderly participants compared to young adults [56]. All the studies related with the sAA activity fluctuations in adulthood, categorised the subjects in age groups and proceed with a comparison between these age groups. In our study we focused in the subjects of one age group only and found a positive association between age and sAA activity in young adults 20 to 39 years old. Finally a negative association of the sAA is observed with the interaction between flow rate and age.
To conclude, in the absence of a stressful stimulus, the flow rate, age as well as the interaction of these two factors affect the secretion of sAA in healthy young adults.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
ACKNOWLEDGEMENTS
Declared none.


