All published articles of this journal are available on ScienceDirect.
Influence of Psychosocial Factors and Habitual Behavior in Temporomandibular Disorder–Related Symptoms in a Working Population in Japan
Abstract
Background:
The symptoms of temporomandibular disorders (TMD) are directly influenced by numerous factors, and it is thought that additional factors exert indirect influences. However, the relationships between TMD-related symptoms (TRS) and these contributing factors are largely unknown. Thus, the goal of the present study was to investigate influences on TRS in a working population by determining the prevalence of TRS, analyzing contributing factors, and determining their relative influences on TRS.
Materials and Methods:
The study subjects were 2203 adults who worked for a single company. Subjects completed a questionnaire assessing TRS, psychosocial factors (stress, anxiety, depressed mood, and chronic fatigue), tooth-contacting habit, and sleep bruxism-related morning symptoms, using a 5-point numeric rating scale. Our analysis proceeded in 2 phases. First, all variables of the descriptor were divided into parts by using an exploratory factor analysis. Second, this factorial structure was verified by using a confirmatory factor analysis with structural equation modeling.
Results:
Of 2203 employees, 362 reported experiencing TRS (16.4%). Structural equation modeling generated a final model with a goodness of fit index of 0.991, an adjusted goodness of fit index of 0.984, and a root mean square error of approximately 0.021. These indices indicate a strong structural model. The standardized path coefficients for “habitual behavioral factors and TRS,” “psychosocial factors and habitual behavioral factors,” “psychosocial factors and TRS,” and “gender and habitual behavior factors” were 0.48, 0.38, 0.14, and 0.18, respectively.
Conclusions:
Habitual behavioral factors exert a stronger effect on TRS than do psychosocial factors.
INTRODUCTION
Temporomandibular disorders (TMD) include a number of clinical conditions that involve the temporomandibular joint (TMJ), the masticatory muscles, or both [1]. It has been reported that 5–12% of the general population has TMD [2, 3]. Since the 1970s, a multifactorial etiology for TMD has been proposed, in which pain and dysfunction result from bio-psychosocial factors [4, 5]. These etiological contributing factors include structural conditions, psychological morbidity, and behavioral problems such as parafunctional habits [6-9].
According to the multifactorial etiology theory, individual factors should be managed at the same time as pathological conditions. However, this approach is sometimes difficult, because each factor may not always be present in all patients. A universal cause of TMD has not been clearly identified to date, further complicating management.
It is thought that trauma or habitual behaviors that burden the TMJ and the masticatory muscles influence the development of TMD. Several studies support an association between bruxism and myofacial pain or TMD [10-12]. Molina et al. [13] observed a positive association between bruxism and TMD in a clinically based case control study. Michelotti et al. [14] reported that the habit of maintaining teeth in contact was a significant risk factor for myofacial pain. Furthermore, Kanehira et al. [15] showed that stress is significantly correlated with parafunctions such as sleep bruxism (SB) and daytime clenching.
Relationships between TMD and psychosocial factors such as stress have also been reported. Many studies suggest that psychosocial factors, including depression, stress, and anxiety, play a role in the predisposition, initiation, and progression of TMD and in the responses of TMD patients to treatment [16-20]. It has also been reported that TMD patients who are more anxious are at greater risk of developing chronic pain than those that are less anxious [21].
Sugisaki et al. [22] reported that the prevalence of TMD-related symptoms (TRS) was higher (approximately 17–18%) among the working population than among the general population (5–12%). They concluded that this difference was attributable to psychological aggravation resulting from duties in the workplace, changes in the work environment, interpersonal relations, and an achievement-oriented climate (e.g., an environment without an employment agreement or one with insufficient output or results).
The Japan Institute for Labour Policy and Training reported that in business establishments, approximately 60% of employees face a mental health problem, and that the number of affected employees had increased by 30% over the past 3 years [23].
While some contributing factors influence TMD symptoms directly, other factors may influence them indirectly. To clarify the relationships between TRS and these contributing factors in a working population, we investigated the prevalence of TRS, the contributing factors that affect TRS, and the interactions between those contributing factors.
METHODOLOGY
This cross-sectional study was performed using an anonymous questionnaire with the approval (No. 325) of the ethics committee of the Tokyo Medical and Dental University, Japan.
Subjects
We recruited 2723 employees of a particular company that had medical checkups at their work-place during the study period (April to October 2008). The company develops, manufactures, and sells electronic parts. The company’s main office and factory are in Tokyo, and it has factories in nearby prefectures. An explanation of the purpose and contents of the study questionnaire was posted at worksites before the study began.
Questionnaires were distributed to all employees along with notification of a medical checkup, and completed questionnaires were collected during the checkup. Of these subjects, 2423 (89%) completed the questionnaire. Written informed consent was not obtained because identification of individuals was not required; answering the questionnaire was considered consent to participate.
Questionnaire
The questionnaire administered is shown in Table 1. Gender, age, and responses to items 1–10 were recorded.
Questionnaire
| Question Items | Abbreviated Form | |
|---|---|---|
| Q1 | If you open your mouth wide, can you fit 3 fingers held vertically in your mouth? | Limited mouth opening |
| Q2 | Do you experience pain in the face, jaw, temple, or in the front of the ear when you open and close your mouth? | Mouth-opening pain |
| Q3 | Can you open your mouth without any deviation? | Mouth-opening deviation |
| Q4 | Do you experience pain in the face, jaw, temple, or in the front of the ear when you eat hard foods such as beef jerky, dried cuttlefish, or octopus? | Chewing-induced pain |
| Q5 | Do you experience stress at work, school, home, or in relationships? | Stress level |
| Q6 | Do you experience anxiety at work, school, home, or in relationships? | Feeling of anxiety |
| Q7 | Do you feel depressed now? | Depressed mood |
| Q8 | Do you feel fatigued even after obtaining rest through sleeping? | Chronic fatigue |
| Q9 | Do you often allow your upper- and lower teeth to make continuous contact during work or at rest? | TCH* |
| Q10 | Do you experience orofacial jaw muscle fatigue or pain when you are awake? | Morning symptoms |
All the questions were evaluated using a 5-grade rating scale: 1) strongly agree, 2) weakly agree, 3) neither agree nor disagree, 4) weakly disagree, 5) strongly disagree. TCH: tooth contacting habit.
Items 1–4, which screened patients for TRS, were developed by Sugisaki et al. [24]. The subjects rated the 4 screening items by using a 5-point numeric rating scale. Sugisaki et al. extracted these 4 items from a 20-item questionnaire administered to dental patients. The sensitivity, specificity, and false-positive rate derived from TRS screening were 0.746, 0.811, and 0.189, respectively. The total score from those 4 items was used for TRS screening with a cut-off value of 8.5: participants with a score of ≥9.0 were assigned to the TRS group, whereas those with a score of ≤8.0 were assigned to the non-TRS group. One item related to joint noise was omitted from the screening questionnaire, as analysis using nonparametric item response theory (Mokken analysis) showed that the validity of the 4 included items was higher than that of the 5 items.
Items 5–8 assessed psychosocial factors, including stress, anxiety, depressed mood, and chronic fatigue, as described by Sugisaki et al., [22]. Validity of those items was not tested. Items 9 and 10 were related to habitual behavior, including tooth-contacting habit (TCH), in which the upper and lower teeth are continuously brought together with minimal force in a nonfunctional context [25], and morning symptoms that presumably result from SB [26-28]. Subjects used the same 5-point numeric rating scale on all 10 items.
Statistical Analysis
The questionnaires returned by 220 respondents were incomplete, and thus excluded from the statistical analysis. Data from the remaining 2203 participants (90.9%) were used for analysis.
Student’s t-tests and chi-square tests were used to compare age, gender, and prevalence of TRS between the 2 groups.
Factors influencing TRS (non-TRS, 0; TRS, 1) were estimated using logistic regression analyses with odds ratio (ORs) and 95% confidence intervals (CI) as measures of association.
Pearson’s correlation coefficient between TRS and factors assessed in questionnaire items 5–10 were analyzed to determine covariates. For each item, participants with a score of ≤ 3 were assigned a value of “0,” whereas those with a score of ≥4 were assigned a value of “1.” Those items were used as covariates and adjusted for age (1-year increments) and gender (man, 0; woman, 1). The covariates were entered into the logistic regression analysis using a stepwise forward technique. P < 0.05 was considered statistically significant.
The structural equation modeling analysis consisted of 2 phases. First, all variables of a descriptor were divided into parts by exploratory factor analysis (EFA). Second, this factorial structure was verified by confirmatory factor analysis (CFA) with structural equation modeling (SEM). EFA was conducted using SPSS (Version 12, SPSS Japan) and CFA was conducted using AMOS (Version 5.0, SPSS Japan). For both phases of these analyses, the 2203 subjects were randomly divided into 2 groups (designated groups “C” and “E”) using an algorithm available in SPSS.
Exploratory factor analysis (EFA) was used to define a separate factorial structure. As an initial step, we attempted to minimize the 10 items. Principal factor analysis (promax solution) was employed as an exploratory factor analysis method, to determine the item groups for the questionnaire using the E-group. As a second step, the hypothesized structural model was generated based on this analysis.
Using data from the C-group, confirmatory factor analysis (CFA) was performed, to verify the hypothesized structural models using SEM.
SEM, which is also known as analysis of covariance structures, or causal modeling, is a statistical technique used for testing and estimating causal relationships using a combination of statistical data and qualitative causal assumptions. SEM includes model fitting, testing, and equating, based on the analysis of covariance structures within the framework of a confirmatory data analytical model, and seeks to test data against a hypothesized or theoretical model [29-31]. Because no single index adequately assessed the fit during SEM, we included 3 indices for goodness-of-fit to evaluate the model: the goodness of fit index (GFI), the adjusted goodness of fit index (AGFI), and the root mean square error of approximation (RMSEA). The model was deemed to be well fit when the GFI and AGFI were > 0.90 and the RMSEA was < 0.05. Furthermore, standardized path coefficients were considered statistically significant when the critical ratio was > 1.96 (P < 0.05).
Where the goodness-of-fit statistics did not reach a satisfactory level, we modified the model according to the modification indices available in the AMOS program.
RESULTS
The characteristics of the subject group are shown in Table 2. Of the 2203 employees, 362 were found to have TRS (TRS group, 16.4%). Women composed a significantly larger proportion of the TRS group than the non-TRS group (P = 0.005), and the mean age of the TRS group was significantly lower than that of the non-TRS group (P = 0.018).
Characteristics of Subjects
TRS: TMD-related symptoms
a achi-square test
b t-test
The cut-off value of the total score for TRS screening (question items 1–4) was 8.5; participants with a score of ≥ 9.0 were assigned to the TRS group, while those scoring ≤ 8.0 were assigned to the non-TRS group.
Logistic Regression Analysis
Correlation coefficients between TRS and items 5–10 are shown in Table 3. As all correlation coefficients were significant, we used all questions as covariates for logistic regression analyses.
Correlations of Questionnaire Items with TMD-Related Symptoms
| Questionnaire Items | Pearson’s Correlation Coefficient (r) | P-Value |
|---|---|---|
| Stress level | 0.128 | < 0.001 |
| Feeling of anxiety | 0.169 | < 0.001 |
| Depressed mood | 0.165 | < 0.001 |
| Chronic fatigue | 0.178 | < 0.001 |
| TCH | 0.107 | < 0.001 |
| Morning symptoms | 0.261 | < 0.001 |
TCH: tooth contacting habit.
The results of the logistic regression analyses are shown in Table 4. Only statistically significant independent variables are shown (P < 0.05). Depressed mood (OR, 1.47; 95% CI, 1.01–2.13), chronic fatigue (OR, 1.96; 95% CI, 1.10–3.51), TCH (OR, 1.91; 95% CI, 1.23–2.95), and morning symptoms (OR, 2.78; 95% CI, 2.19-3.52) were found to be significant factors contributing to the manifestation of TRS.
Logistic Regression Analysis
| OR | 95% CI | P-value | |
|---|---|---|---|
| Depressed mood score 1–3 score 4.5 |
1 1.47 |
1.01 – 2.13 | 0.043 |
| Chronic fatigue score 1–3 score 4.5 |
1 1.96 |
1.10 – 3.51 | 0.023 |
| TCH score 1–3 score 4.5 |
1 1.91 |
1.23 – 2.95 | 0.004 |
| Morning symptoms score 1–3 score 4.5 |
1 2.78 |
2.19 – 3.52 | > 0.001 |
OR: odds ratio, CI: confidence interval, TCH: tooth contacting habit
Exploratory Factor Analysis
Characteristics of the C-group and E-group are shown in Table 5. There were no significant differences between these groups with respect to age, or the prevalence of TRS.
As a result of factor analysis of the E-group, 3 factors were extracted (Table 6). It was assumed that items 5–8 comprised the first factor, items 1-4 comprised the second factor, and items 9 and 10 the third factor. We named the first, second, and third factors as “psychosocial factors,” “TRS,” and “habitual behavioral factors,” respectively. A hypothesized structural model including the observed variables was generated from these results (Fig. 1).
Questionnaire Items and Factor Loading of Factor Analysis on the E-group
| Questionnaire Item | Factor | |||
|---|---|---|---|---|
| 1st | 2nd | 3rd | ||
| Psychosocial factors | Q5 Stress level | 0.919 | 0.012 | -0.134 |
| Q6 Feeling of anxiety | 0.912 | 0.033 | -0.113 | |
| Q7 Depressed mood | 0.736 | -0.073 | 0.186 | |
| Q8 Chronic fatigue | 0.490 | 0.045 | 0.215 | |
| TRS | Q2 Mouth-opening pain | -0.055 | 0.731 | 0.000 |
| Q1 Limited mouth opening | -0.009 | 0.581 | -0.079 | |
| Q3 Mouth-opening deviation | 0.045 | 0.554 | -0.034 | |
| Q4 Chewing-induced pain | 0.038 | 0.471 | 0.017 | |
| Habitual behavior factors | Q10 Morning symptoms | 0.026 | 0.232 | 0.344 |
| Q9 TCH | -0.017 | -0.072 | 0.338 | |
| Proportion of variance (%) | 28.9 | 12.3 | 1.8 | |
TRS: TMD-related symptoms, TCH: tooth contacting habit
Based on the results, we identified three latent variables; (1) Psychological factor, (2) Symptoms of TMD, and (3) Habitual behavioral factors.

Hypothesized structural model including observed variable e1 to e12 are error variables. TRS: TMD-related symptoms, TCH: tooth contacting habit.
Confirmatory Factor Analysis
We performed confirmatory factor analysis using SEM to investigate the hypothesized structural model. Significant standardized path coefficients in the final model are shown in Fig. (2). The fit indices of the final model were as follows: GFI = 0.991, AGFI = 0.984, and RMSEA = 0.021. These indices indicated a strong structural model.
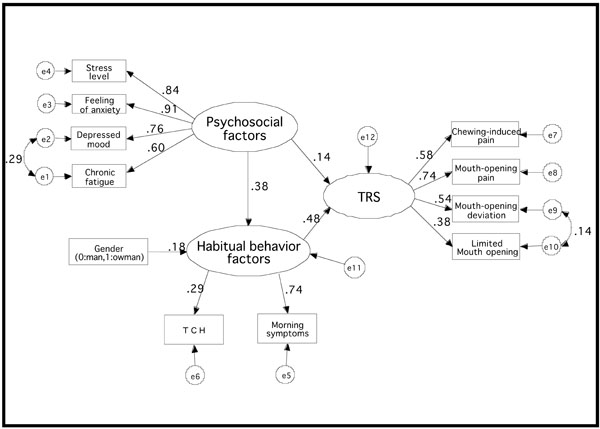
Standardized path coefficients GFI = 0.991, AGFI = 0.984, RMSEA = 0.021; e1 to e12 are error variables. TRS: TMD-related symptoms, TCH: tooth contacting habit.
The standardized path coefficients for “habitual behavioral factors and TRS,” “psychosocial factors and habitual behavioral factors,” “psychosocial factors and TRS,” and “gender and habitual behavior factors” were 0.48, 0.38, 0.14, and 0.18, respectively. These standardized path coefficients were statistically significant (Table 7).
Critical Ratio and P-Value in Standardized Path Coefficients
| Critical Ratio | P-Value | |
|---|---|---|
| Habitual behavior factors ⇒ TRS | 4.187 | < 0.001 |
| Psychosocial factors ⇒ TRS | 2.190 | 0.029 |
| Psychosocial factors ⇒ Habitual behavior factors | 8.856 | < 0.001 |
| Gender ⇒ Habitual behavior factors | 4.819 | < 0.001 |
TRS: TMD–related symptoms.
Standardized path coefficients are significant statistically when the critical ratio is greater or equal to 1.96.
DISCUSSION
Women formed a significantly larger proportion of the TRS group (19.1%) than the non-TRS group (13.3%). It has been reported that patients requiring treatment for TMD are predominantly women [32]. Thus, our data confirm the widely reported relationship between gender and painful TMD symptoms [33-35].
Depressed mood, chronic fatigue, TCH, and morning symptoms were found by logistic regression analyses to be significant factors contributing to TRS. Manfredini et al. [36] reported that pain-related disability was strongly correlated with depression and somatization levels in a multicenter questionnaire study. Korszun et al. [37] showed that 42% of a group of patients with chronic fatigue syndrome or fibromyalgia reported temporomandibular disorders. In our study, ORs of depressed mood and chronic fatigue were 1.47 and 1.96, respectively. This suggests that when both factors are prevalent, the risk of TRS is increased (OR = 2.88).
Several studies support an association between bruxism and myofacial pain or TMD [12-14]. It is possible that SB causes TMJ pain and/or jaw muscle pain in the morning [38] and that morning symptoms represent an important element in diagnosing SB when a patient seeks advice relating to tooth grinding or clenching [26]. Dubé et al. [39] also used morning orofacial jaw muscle fatigue to diagnose SB. It has been shown that mandibular condyle is displaced and TMJ stress is increased by the occlusal force [40-43]. Baba et al. [44] reported that the frequency of clicking increased with the severity of subjectively evaluated SB.
In 2006, Sato et al. [25] first described daytime light clenching TCH. TCH was defined as a habitual behavior in which the upper and lower teeth are continuously brought together with minimal force in a non-functional situation, i.e., contact but not clenching. We considered TCH a risk factor for TMD, since the activity of the masticatory muscles in the intercuspal position was shown to be higher than when at rest [45]. Using a radio wave-activated wrist vibrator, Chen et al. [46] showed that patients with myogenous pain exhibit nearly 4 times more non-functional tooth contact during the daytime than healthy controls. Michelotti et al. [16] also reported that the habit of maintaining tooth contact was a significant risk factor for myofacial pain. Given that the average total time of functional tooth contact during chewing or swallowing is only 17.5 min/day [47], it is thought that continuous non-functional tooth contact causes overload of the TMJ and the masticatory muscles. In fact, it has been shown that the association between clenching and muscle pain is either due to damaged muscle fibers or to a reduction in blood supply to these fibers, as the perfusion of the masseter was significantly lowered [40, 48-52] during clenching. Therefore, TCH also affected the onset, persistence, and aggravation of TMD [22, 25].
Our SEM analysis showed that gender influenced habitual behavior factors and suggested an association between TRS, psychosocial factors, and habitual behavior factors.
Habitual behavior influenced TRS directly, whereas psychosocial factors influenced habitual behavior but did not directly influence TRS. This shows that added force on the TMJ and masticatory muscles has a direct influence on TMD.
Psychosocial factors such as anxiety and depressed mood act as secondary factors that can elevate habitual behaviors such as SB and TCH. Pingitore et al. [53] found that the total score of life stress events was significantly and positively correlated with bruxism in 125 dental patients. Kanehira et al. [15] reported that stress was significantly correlated with parafunctions such as SB and daytime clenching. Furthermore, Manfredini et al. [54] showed that wake-time clenching appears to be associated with psychosocial factors. Regarding the difference between men and women, SB is reportedly weakly associated with some aspects of job stress in the male but not in female the Japanese working population [55]. In this study, because the standardized path coefficient from psychosocial factors to habitual behavioral factors was significant, we suggest that psychosocial factors are associated with SB and TCH. According to our previous study of the same workforce, TRS was associated with an increase in both anxiety and habitual behaviors, similar to the way that morning symptoms are associated with SB and TCH [22].
Various factors such as working hours, and working environment, influence psychosocial factors. We assessed visual display terminal (VDT) work in the working environment because several reports suggest that VDT use influences musculoskeletal symptoms. VDTs are now used in many workplace situations, and the number of individuals exposed to these systems has consequently increased in recent years. Nakazawa et al. [56] reported that the likelihood of developing physical symptoms such as headache, neck pain, back pain, and eye strain increased when daily exposure to VDTs exceeded 3 h, and that mental and sleep disorders could be prevented by restricting the use of VDTs to ≤ 5 h/day. In addition, prolonged and uninterrupted daily VDT usage causes eye strain and musculoskeletal pain, both of which are associated with deterioration of mental health [57, 58]. Because TMD is considered a symptom of the musculoskeletal system, it is conceivable that VDT use has an indirect influence on TMD.
These observations indicate that correction of habitual behaviors is important for the treatment of TMD, because those factors influence TRS directly. However, it is difficult to control SB because its cause is unknown. Therefore, we suggest that the control of TCH is an important goal in the effective treatment of TMD.
We advocate the use of behavior modification to control TCH. Behavior modification is the use of empirically demonstrated techniques to improve behavior. Behavior modification targets behaviors that can be objectively measured and aims to control these behaviors by reinforcing adaptive behavior and/or reducing maladaptive behavior. This process consists of behavioral assessment to measure the target behavior and subsequent modification to change that behavior. We propose that TCH can be effectively controlled using a time sampling method for behavioral assessment and the habit reversal method for modification. These procedures consist of 3 steps. The first step is “motivation strategy,” in which the patient confirms habitual behavior using reminders such as tags, stickers, and timers. The second step entails “awareness training” and “competing response training,” in which the patient performs a substitute action in place of the adverse habitual behavior (for example, taking a deep breath) just after becoming aware of it, via a reminder. After performing the behavior modification procedures, the patient will feel less jaw muscle strain. The final step is “reinforcement,” in which the patient increases the frequency of noticing the behavior by performing the first and second steps repeatedly.
Our results indicate that habitual behaviors such as SB and TCH are contributing factors that directly influence TRS, whereas psychosocial factors such as stress, anxiety, and depressive mood are secondary factors that elevate habitual behaviors. In the future, it will be necessary to consider habitual behavior factors other than SB and TCH. In addition, factors that influence TRS and aspects of the work environment that lead to psychosocial deterioration should be examined separately.
The subjects in the current study may not be entirely representative of the general population, as they were all employees of single company. Future investigations involving employees from various type of industry, of the general population, will be of further interest.
CONCLUSIONS
The relationship between TRS and contributing factors such as psychosocial and habitual behaviors was reviewed using SEM in a working population. We showed that habitual behavioral factors such as stress, anxiety, depression, and fatigue, had a stronger effect on TRS than psychosocial factors such as SB and TCH. Further, psychosocial factors lead to the development of habitual behaviors, and these habitual behaviors lead to the development of TRS. Future studies should consider other factors such as occlusion, and additional habitual behaviors and work environments.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGEMENTS
We thank the subjects who participated in this study.


