All published articles of this journal are available on ScienceDirect.
Investigation of Peri-Implant Bone Healing Using Autologous Plasma Rich in Growth Factors in the Canine Mandible After 12 Weeks: A Pilot Study
Abstract
Introduction:
Faster reconstruction of patients’ masticatory systems is the aim of modern dentistry. A number of studies have indicated that application of growth factors to the surface of a dental implant leads to accelerated and enhanced osseointegration. The objective of the present study was to investigate the effect of plasma rich in growth factors on peri-implant bone healing.
Materials and Methods:
For the purpose of this study, two healthy, mixed-breed canines were selected, and the premolars were extracted from both sides of the mandible. Three months after premolar removal, 12 implants, each 5 mm in diameter and 10 mm in length, were placed in osteotomy sites on both sides of the mandible. Prior to placement, plasma rich in growth factors was applied to the surfaces of six implants, while the other six were used without plasma rich in growth factors. The implants were removed after 12 weeks along with the bone surrounding the sites using a trephine bur. One mesiodistal section containing the surrounding bone from each implant block, 50 µm in diameter, was prepared for histologic and histomorphometric investigation with an optical microscope.
Results:
The sites with implants treated with plasma rich in growth factors showed more bone-to-implant contact compared to control sites. Also, higher values for bone trabecular thickness and bone maturity were recorded for the PRGF-treated sites than for the control sites.
Conclusion:
Application of plasma rich in growth factors to the surface of an implant may enhance the bone healing process as well as bone-to-implant contact, thereby helping to achieve faster osseointegration.
INTRODUCTION
Over the past few decades, the most common remedy for edentulous mandibles was reconstruction by removable dentures, which caused functional and esthetic problems in addition to inconvenience for the patients. Nowadays, dental implants are frequently used to support fixed prostheses or removable overdentures [1]. An ongoing problem with implant treatment, however, is the time needed for the peri-implant bone to heal; loading of the implants is usually delayed and patients must continue to be edentulous for long periods. To address these problems, many efforts have been directed toward improving the macroscopic and microscopic structures of implant surfaces in an attempt to accelerate and enhance osseointegration; these investigations have yielded positive results. In addition, tissue engineering principles have been invoked by other scientists in the use of biological constituents such as growth factors or bone-specific proteins, including bone morphogenetic proteins, on implant surfaces or inside localized alveolar bone defects to enhance the rate of bone formation. Numerous studies have been performed on platelet-derived growth factors [2-6]. These studies have also demonstrated successful results.
The idea behind these studies is that growth factors are capable of creating an active and dynamic environment on the implant surface that, in turn, can encourage the creation of new peri-implant bone [7, 8]. Investigations in human, animal, and in vitro models have shown that the application of growth factors such as platelet-rich plasma (PRP) or plasma rich in growth factors (PRGF), either directly to autogenous bone or allografts, xenografts, or alloplasts or inside bone defects, may lead to faster healing rates and enhanced bone formation [9-19]. PRGF always and exclusively uses a patient’s own autologous proteins, which are prepared at the same time they are used. PRGF contains platelet and plasma growth factors involved in the repair process; it also contains sticky plasma proteins such as fibrin, fibronectin, and vitronectin, among others [6, 18]. Some researchers have even reported that the high concentration of transforming growth factor β (TGF-β) present in platelets via PRP can give rise to better repair of the emerging tissue and to enhanced bone reconstruction. They claim that the growth factors released by platelets play an important role in improving the bone response and in the transformation of marrow bone cells into osteoblasts [20].
In contrast, some researchers have challenged the application of growth factors to bone tissue for enhancing bone reconstruction [21-27]. Giovanini et al. [25] created four 16-mm2 bone defects in the calvaria of 21 rabbits. The surgical defects were treated with either particulate autograft, particulate autograft mixed with PRP, or PRP alone. The histomorphometric results demonstrated intensive deposition of fibrous tissue, while bone deposition was hindered in PRP groups. These results coincided with higher values of TGF-β on the PRP sample. The authors explained that the higher levels of TGF-β associated with the expression of collagen III, a smooth muscle actin, on defects treated with PRP suggest that this biomaterial induces an effect that can be considered similar to that of a fibroproliferative disorder. Arpornmaeklong et al. [26] compared the effects of PRP and bone morphogenetic protein 2 in vitro. They reported that PRP showed a dose-dependent stimulation of cell proliferation while reducing alkaline phosphatase activity and calcium deposition in the culture. Bone morphogenetic protein 2 led to an opposite cell response and induced the highest alkaline phosphatase activity and mineral deposition. These data suggest that PRP inhibited osteogenic differentiation of marrow-derived preosteoblasts in a dose-dependent manner. The authors further claimed that the useful clinical effects observed in applications of PRP may have been the result of one of the following two causes: (1) formation of an autologous fibrin gel, which may have given rise to stabilization of the graft material and the blood coagulate due to its adhesive strength; or (2) the strong mitogenic properties of PRP for soft tissue cells, which improve reconstruction treatments because of the associated faster repair process and reduced likelihood of wound dehiscence.
Because of the controversy regarding the effectiveness of growth factors in enhancing peri-implant bone healing, the present study sought to evaluate the effects of autogenous PRGF on the quantity and quality of peri-implant bone reconstruction in the canine mandible.
MATERIALS AND METHODS
Two male mixed-breed canines (each around 25 kg) were selected for this split-mouth study. Animal selection, management, and preparation and surgical protocols were adopted from a study of Wikesjo et al. [28] and were in accordance with the instructions of the Medical Ethics and Institutional Animal Care Committee of Isfahan University of Medical Sciences, Isfahan, Iran.
Surgical Procedure
The animals used in this study were weighed 3 months after extraction of the premolars on both sides of their mandibles. A mixture of 10% ketamine hydrochloride (40 mg/kg) and 2% xylazine (5 mg/kg) (Alfasan International, Woerden, The Netherlands) was administered by deep muscular injection to induce anesthesia. An intubation tube containing a combination of oxygen and 15% halothane was used to maintain anesthesia. Immediately after the anesthetic took effect, blood samples were taken from the tibia and transferred to a centrifuge.
While the blood sample was being prepared, the surgeon made crestal incisions in the edentulous area on each side of the mandible to create three osteotomy sites, each 5 mm in diameter and 10 mm in length. The surgeon then placed three OIC implants (Osteo Implant Corporation, New Castle, PA, USA) in a submerged technique inside the osteotomy sites in the right mandible. The edges of the reflected flap were then sutured using an interrupted technique. On the left side of the mandible, PRGF was injected into the prepared sites, and three more implants were first immersed in PRGF and then placed into the osteotomy sites.
The same procedure was repeated in the other dog, except that the implants that had been immersed in PRGF were placed in the right side of the mandible.
During the first 12 weeks after implant placement surgery, infection control was accomplished by daily topical application of 2% chlorhexidine solution coupled with administration of a broad-spectrum antibiotic (a combination of penicillin G 10,000 IU/kg and streptomycin 100 mg/kg, Hayyan Pharmaceutical Co, Tehran, Iran). The animals were fed soft food supplemented with vitamins throughout the study period [29].
PRGF Preparation
The creation and application of PRGF followed the protocol of Anitua et al. [6]. A blood sample of 20 mL was taken from the saphenous vein in the tibia and directly transferred to the PRGF System (BTI Biotechnology, San Antonio, Spain). For PRGF preparation, a one-stage centrifuge was used at a low speed (460g) for 15 to 20 minutes at room temperature. Sterile tubes containing a 38% sodium citrate solution were used to prevent blood coagulation. For activation and aggregation of platelets, 50 mL of 10% calcium chloride was used for each milliliter of PRGF. After the blood sample had been centrifuged and the plasma had been separated from the red and white blood cells, the plasma in each tube was fractionated into three distinct fractions. Fraction 3, designated as PRGF, is the layer immediately over a layer of red blood cells and contains a considerable amount of platelet-derived growth factors [30].
Histologic Preparation
Twelve weeks after the implants had been placed, biopsy blocks containing the implants and the surrounding bone were removed using a trephine bur (no. 8; Meisinger, Neuss, Germany). Each specimen was kept for 10 days in a coded package containing 10% formalin solution and then dehydrated with alcohol. The specimens were then transferred into a transparent cold-setting acrylic resin (Fig. 1). The ground histologic sections were prepared to obtain mesiodistal sections 50 µm thick and further prepared according to the Donath method [31]. The sections were sent for examination by an oral pathologist with an optical microscope (BX51; Olympus Co, Tokyo, Japan). The parameters recorded for each section included: (1) inflammation rate (scored as follows: 0, none or few inflammatory cells, no reaction; 1, < 25 cells, mild reaction; 2, 25 to 125 cells, moderate reaction; 3, > or = 125 cells, severe reaction [31,32]); (2) type of osseous tissue generated (eg, lamellar bone, woven bone); (3) thickness of bone trabeculae; and (4) bone-to-implant contact (BIC) percentage. BIC is a histologic concept traditionally evaluated by calculating the amount of the implant surface directly attached to mineralized bone without the interposition of soft connective tissue. Three sections of each specimen were examined, and the average of the three measurements was reported as the BIC for that implant. The predominant cell type, calcification, and thickness of the fibrous connective tissue were also recorded.

(Left) Control sample and (right) PRGF-treated sample within the cold resin (40X magnification).
RESULTS
In this experiment, healing proceeded without difficulty in both animals; no infections or implant or bone exposures were observed in any of the implant sites.
A comparison of PRGF-treated and control sites revealed identical inflammation rates.
Bone maturity was greater in the PRGF group than in the control group (Figs 1 to 4, Table 1). Moreover, it was possible to see lamellar bone around the PRGF-treated implants under polarized microscopy (Fig. 3).

Newly formed bone at (A) low magnification (40X) and (B) high magnification (100X). It is possible to see newly formed bone (woven bone, WB, and lamellar bone, LB) and the presence of well-ordered trabeculae with marginal osteoblastic activitiy in the PRGF section compared to the control (basic fuchsin– toluidine blue). C = connective tissue; OB = osteoblast; BM = bone marrow; T = trabeculae.
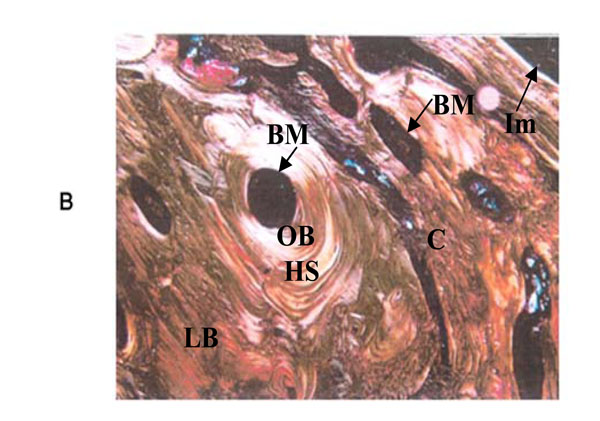
PRGF section at 12 weeks. It is possible to see lamellar bone around the PRGF-treated implant with polarized microscope (100X). HS = Haversian system; BM = bone marrow; LB = lamellar bone; OB = old bone; C = connective tissue; Im = implant.
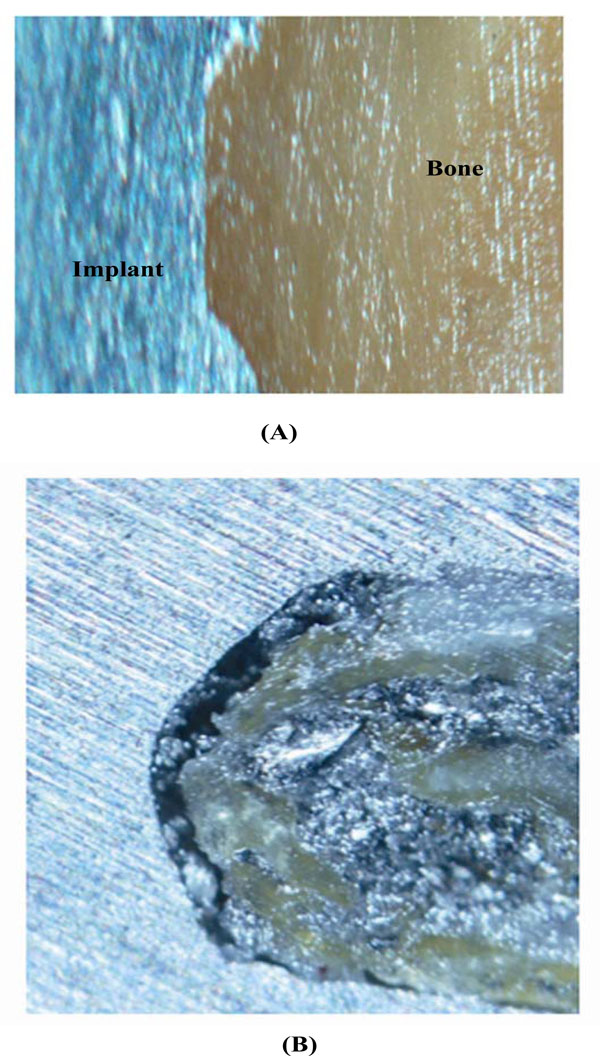
Comparison of BIC (length of direct bone-to-implant contact as a percengae of the implant surface) in (A) PRGF-treated and (B) control samples. In the PRGF-treated sample, almost complete contact of the bone within the thread is seen (basic fuchsin–toluidine blue; original magnification 100X).
Thickness of Bone Trabeculae at Different Sites
| Trabecular Thickness | |||
|---|---|---|---|
| Study Group | I | II | III |
| PRGF | 5 (83.3%) | 1 (16.6%) | 0 |
| Control | 4 (66.6%) | 2 (33.3%) | 0 |
PRGF = plasma rich in growth factors. Definitions of trabecular thickness grades: Grade I = Thicker than 60 µm (thick); grade II = 20–60 µm (medium); grade III = thinner than 20 µm (thin).
The thickness of the bone trabeculae was greater in the PRGF-treated sites than in the control sites (Table 2). Fig. (2a and 2b) show the presence of well-ordered trabeculae with marginal osteoblastic activity in the PRGF section, compared to the control.
Maturity of Regenerated Bone at Different Sites
| Bone Type | |||
|---|---|---|---|
| Study Group | I | II | III |
| PRGF | 4 (66.6%) | 2 (33.3%) | 0 |
| Control | 2 (33.3%) | 4 (66.6%) | 0 |
PRGF = plasma rich in growth factors. Definitions of bone type: Grade I = lamellar bone; grade II = lamellar and woven bone; grade III = woven bone.
Comparisons were also made between the experimental and control groups in terms of BIC percentage. BIC percentage was higher in the PRGF group (Table 3, Fig. 4).
Means and Standard Errors of BIC Percentage for the Different Treatments at 12 Weeks
| Treatment | BIC % (Mean ± SE) |
|---|---|
| PRGF Control |
59.83 ± 3.1 45.67 ± 3.7 |
BIC = bone-implant contact; PRGF = plasma rich in growth factors.
DISCUSSION
Despite controversy regarding their effectiveness, growth factors are frequently used in medicine and dentistry. In dentistry, they are used as PRP and PRGF to treat problems such as periodontal infrabony defects, peri-implant bone defects, and denuded dental roots, and in procedures including maxillary sinus augmentation and osteodistraction [32]. However, more recently, the efficacy of growth factors in bone tissue reconstruction has been seriously challenged [21-25].
The present study was designed on the basis of histologic and histometric evaluations to investigate the effect of PRGF on the healing process of peri-implant bone in the canine mandible. The parameters used to evaluate bone healing consisted of inflammation rate, the maturity of newly generated bone, BIC percentage, and bone trabecular thickness.
The PRGF and control treatments exhibited similar rates of inflammation over the 12 weeks following surgery. The severity of inflammation was evaluated in most cases as grade 0 (no inflammation). This finding is in agreement with the findings reported by Fontana et al. [15].
The maturity of newly regenerated bone was investigated during the same 12-week period. A greater amount of mature bone was observed in the PRGF-treated sites than in the control sites (Table 1). None of the samples in either group was evaluated as grade III (woven bone). This indicates that remodeling is a function of time, as also reported by Philippart et al. [14]. However, Choi et al. [23] reported different results in their canine study. Their fluorescence microscopic examinations revealed that bone grafts containing PRP exhibited delayed remodeling. Zechner et al. [8] reported the time course of local bone formation following the application of PRP during implant placement in minipigs. The animals were sacrificed at 3, 6, and 12 weeks, and undecalcified ground sections were prepared. They reported significant improvement in the 6-week specimens but not in the 12-week specimens. These differences in time and dose dependence may be related to the use of different animals and quality/quantity of PRGF obtained from the animals.
The data in Table 2 show that thicker bone trabeculae were observed around the PRGF-treated implants than around the control implants. This finding is in agreement with the results reported by Wojtowicz et al. [11] and Fuerst et al. [3].
In this experiment, application of PRGF to implant surfaces gave rise to faster healing of the bone surrounding the implant and enhancement of BIC compared to the control treatment (PRGF treatment = 59.83% ± 3.1%, control = 45.67% ± 3.7%) (Table 3). This is in agreement with the findings of Fontana et al. [15], Kim et al. [16], and Fuerst et al. [3]. In their investigation with minipigs, Fuerst et al. [3] placed implants on both sides of the mandible. On one side, the implant was treated with platelet-derived growth factors before placement, while the other was placed untreated. After a period of 4 to 8 weeks, the authors measured BIC percentage at both types of sites. During the experimental period, BIC at the surface of growth factor–treated implant surfaces exhibited a mean value of 55.3%, while the mean control BIC was 38.91%. The authors also reported that application of platelet-derived growth factors could be used to strengthen the anchorage of mandibular implants.
While the observation of an advantage associated with growth factor treatment agrees with the findings of the present study, it contradicts those reported by Weibrich et al. [22], Schlegel et al. [13], Jensen et al. [24], and others. Weibrich et al. [22] carried out an experiment in rabbits to investigate the effect of platelets present in PRP on peri-implant bone reconstruction. They reported no differences in terms of BIC between the experimental and control groups. They also reported that only a very limited range of platelet concentration (about 1 million platelets/µL) may have positive effects on bone reconstruction, such that any concentration below or above this value may have an inhibitory effect. They found no tangible differences between the two groups with respect to BIC percentage. Schlegel et al. [13] reported no considerable effect for PRP when applied for conditioning the implant bed. Jensen et al. [24] investigated the use of PRP with frozen or processed allografts and titanium implants covered with hydroxyapatite and reported no enhancements in osseous regeneration or implant stability.
The following may be claimed as possible reasons for the controversial results reported for the effects of platelet-derived growth factors:
- The commercial PRP or PRGF preparation systems available commercially may use different amounts of living platelets as a basis for platelet concentrate. Studies have reported that the reproduction and differentiation of mesenchymal stem cells are direct functions of platelet concentration; hence, the differences in the results reported [33].
- The growth factors present in alpha granules will be released rapidly; thus, they may lose their effectiveness if the PRGF sample is not prepared in an environment containing an anticoagulant [34].
- Differences in the amounts of animal and human blood samples taken may result in differences in platelet concentrations and, as a result, in the growth factors derived [3].
- Differences in the numbers of specimens and evaluation methods employed may cumulatively affect study results.
- Animal and clinical studies have shown different results with the use of PRP and its effect on bone healing. This could be a result of the differences between species; that is, differences between species in growth factor concentrations or variations in the presence of growth factors between the various PRPs [35].
A major limitation of this study is in its use of only two animals. Although many samples were obtained from the 12 implants placed, it is possible that subject-related factors influenced the findings, since all samples came from only two animals. Future animal studies involving PRPs and implant placement should employ a larger number of test animals to confirm the present results.
CONCLUSION
Based on the findings of the present study, it may be concluded that application of PRGF to peri-implant bone may enhance the healing of bone surrounding implants, including bone-to-implant contact. Application of this method may, therefore, be effective in enhancing the rate of osseointegration. Future studies of larger numbers of animals should be conducted to confirm the present findings.
ACKNOWLEDGEMENT & CONFLICT OF INTEREST
There are no conflicts of interest and no financial relationships related to any products involved in this study.


