All published articles of this journal are available on ScienceDirect.
Mast Cells and Angiogenesis in Oral Malignant and Premalignant Lesions
Abstract
Mast cell contribution to neoangiogenesis during tumorigenesis in oral squamous cell carcinoma is not determined yet. Objectives: To associate numerical mast cell density (MCD) to numerical microvessel density (MVD) during the progression of oral leukoplakia without dysplasia and leukoplakia with dysplasia to squamous cell carcinoma (OSCC). Materials and methods: MVD was analysed immunohistochemically (mouse monoclonal anti-human CD34) in 49 paraffin-embedded specimens, 35 OSCCs, 9 leukoplakias and 5 normal oral tissues. Toluidine blue counterstaining revealed mast cells. MCD and MVD were assessed at the same optical field. Results: MVD increased between: normal oral mucosa, dysplasia (p=0.004), OSCC (p=0.001), leukoplakia and OSCC (p=0.041). MCD increased between: normal oral mucosa, dysplasia (p=0.003), OSCC (p=0.000), leukoplakia and OSCC (p=0.007). MVD was found to depend on MCD (p=0.000) in a percent 28.3% (power curve fit model). Conclusions: Mast cells are attracted at the lesion site and may turn on an angiogenic switch during tumorigenesis in OSCC.
INTRODUCTION
Mast cells are phylogenetically old, highly granulated cells, already known by their key role in type I hypersensitivity reaction [1]. They are the main effector cells in IgE-associated disorders but also seem to play important roles in acquired or innate host reactions [2]. Mast cells can release numerous pro-inflammatory, immunoregulatory and angiogenic molecules through different stimulation pathways [3]. The activation of mast cells has been proved to have many biological consequences such as mitogenesis, extracellular matrix degradation, angiogenesis and augmentation of microvascular hyperpermeability [4] and recruitment of inflammatory cells including macrophages. It is already known that neoangiogenesis is required for the growth and spread of tumors [5]. Increased angiogenesis has been associated with neoplastic progression, metastasis and outcome in several studies and in a number of malignancies [6-12]. Recent data suggest that the acummulation of mast cells around the tumor margins and their release of potent pro-angiogenic and angiogenic factors may represent a tumor-host interaction which probably favors tumor progression [13-15]. The accummulation of mast cells is usually estimated by counting the mast cell density, which is the number of mast cells per optical field in tissue sections [16]. The contribution of mast cells, however, to angiogenesis during the progression from oral leukoplakia without dysplasia to oral leukoplakia with dysplasia or oral squamous cell carcinoma is not clear yet, due to conflicting results within the literature [14, 17].
The purpose of the current study was to examine the relationship between mast cells, angiogenesis and the histological progression from normal oral tissues through leukoplakia lesions with various degrees of dysplasia to OSCC. We analyzed, therefore, mast cell density and microvessel density in oral leukoplakia without dysplasia, oral leukoplakia with mild moderate or severe dysplasia dysplasia and oral squamous cell carcinoma.
MATERIALS AND METHODS
Tissue Specimens
Fourty-nine (49) formalin-fixed, paraffin-embedded tissue specimens obtained from oral biopsies, were retrieved from the archives of the histopathology laboratory from the Department of Oral Medicine and Maxillofacial Pathology. The tissue specimens were: 30 oral squamous cell carcinomas, 7 leukoplakias without dysplasia and 12 leukoplakias with dysplasia (4 with mild, 4 with moderate and 4 with severe dysplasia). Five samples from normal oral tissue were used as a control group.
In order to focus on the early stages of head and neck cancer, the selection criteria included: Newly diagnosed patients, who visited the Department of Oral Medicine and Maxillofacial Pathology during the period from 2000 to 2007 and invasive squamous cell carcinomas characterized as T1N0M0 or T2N0M0, according to the TNM staging system. The TNM stage was determined after a complete radiographic examination of the patients and the surgical excision of the lesions. The diagnosis was based on the clinical examination and the histopathologic analysis of the specimens. The clinical diagnosis of leukoplakia was given to any white patch or plaque that could not be characterized clinically or pathologically as any other disease or condition such as lichen planus, chronic cheek bite, frictional keratosis, tobacco keratosis, nicotine stomatitis, leukoedema and white sponge naevus. Histopathologically leukoplakia lesions were characterized by a thickened keratin layer of the surface epithelium and/or a thickened spinous layer [18]. The diagnosis of epithelial dysplasia was based on the criteria described in the report from the WHO international collaborating center for oral precancerous lesions [18-20].
The recorded clinical and histopathologic data included age, gender, clinical lesion stage, site of the lesion and histopathologic differentiation. The study was conducted according to the Helsinki declaration.
From each paraffin block, two tissue sections, 4 µm thick each, were placed on a slide.
Determination of Numerical Microvessel Density
In order to assess microvessel density, immunohisto-chemistry staining was conducted. Upon one out of the two tumor sections on each slide, a mouse monoclonal anti-human CD34 Class II antibody (clone QBEnd-10) (Dako Cytomation) (Glostrup, Denmark) was applied. The second tissue section in each slide was stained with anti-mouse IgG1 antibody {(mAb, Mouse IgG1 (MOPC21), Sigma-Aldrich Co., Steinheim, Germany)} to serve as an internal negative control. Formalin-fixed, paraffin-embedded human sarcoma Kaposi tissue known to highly express CD34 was used as an internal positive control for the measurements of CD34 expression. Tissue samples were deparaffinized in xylene and rehydrated with graded alcohols. Incubation of the slides in 1% hydrogen peroxide (H2O2) with methanol for 30 minutes blocked the endogenous peroxidase activity. The slides were then washed with TBS (Triphosphate buffered saline). To retrieve antigen epitopes, tissue sections were heated in citric-acid buffer, at pH 6.7, for 10 minutes in a microwave oven. The slides were then left to cool at room temperature for 20 minutes. The anti-CD34 antibody was diluted (1:50) and applied on the tissue sections for one hour at room temperature. Immunostaining was then performed using the Envision system (Envision HRP, Mouse/Rabbit detection system) (Dako, Glostrup, Denmark). Afterwards diaminobenzidine chromogen (DAB chromogen) was applied.
Counterstaining with Toluidine Blue for Determination of Numerical Mast Cell Density
The tissue sections, after the immunohistochemical staining, instead of being counterstained with hematoxylin, they were counterstained with toluidine blue. The slides were quickly dipped 15 times in a 0.1% tolouidine blue solution and then were washed in tap water to remove excess chromogen. Afterwards, the slides were quickly dipped once in a solution containing 70% alcohol and 0.5% HCl and were dried by firmly pressing them once, against a suitable absorbent paper. They were then washed twice, by 12 dipps each time in xylene and were mounted with entalan. Toluidine blue reveals the mast cells [21, 22].
Using this technique, mast cells and microvessels can be observed together at the same slide (Fig. 5). The number of mast cells and microvessels were then counted together, at the same optical field.
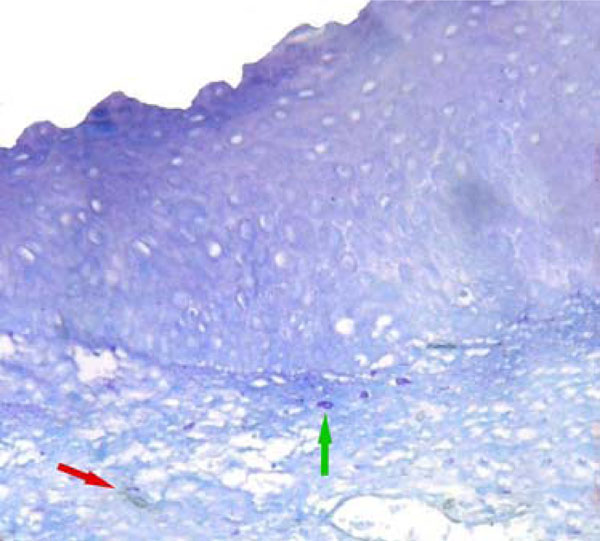
MVD (red arrow) and MCD (green arrow) were low in normal oral epithelium. (CD34 & toluidine staining, original magnification X200).
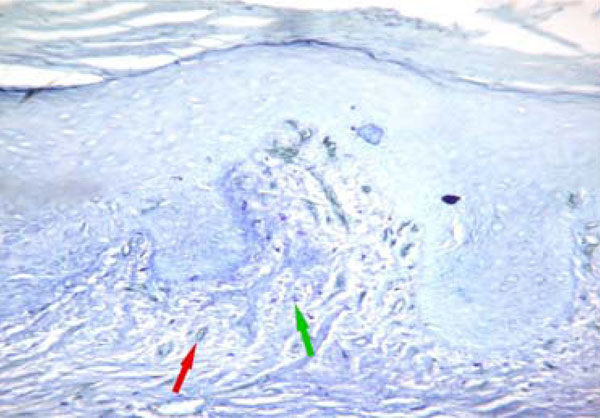
MVD (red arrow) and MCD (green arrow) were low in oral leukoplakia without dysplasia similar to those of normal oral epithelium. (CD34 & toluidine staining, original magnification X200).

MVD (red arrow) and MCD (green arrow) were significantly increased in oral leukoplakia with dysplasia. (CD34 & toluidine staining, original magnification X200).
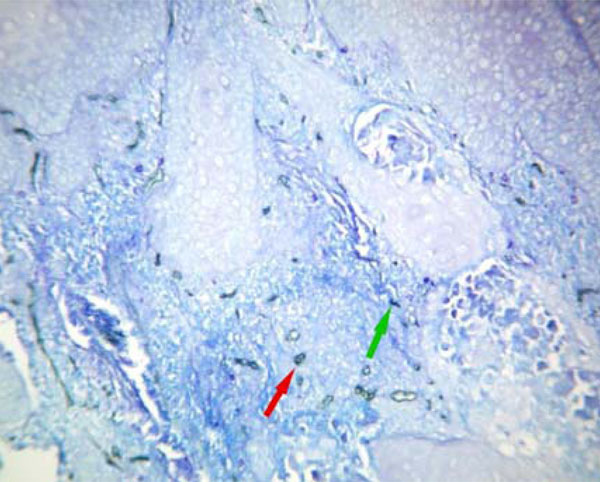
MVD (red arrow) and MCD (green arrow) were significantly increased in oral squamous cell carcinoma. (Original magnification X200).

Mast cell (green arrow) and microvessel (red arrow) revealed with toluidine blue counterstaining (Original magnification X400).
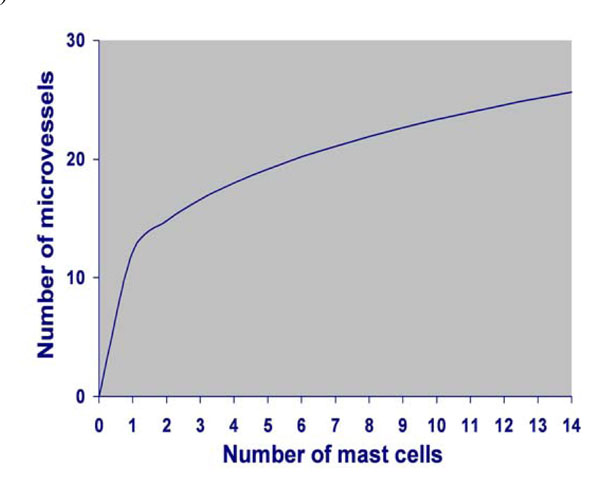
Power curve fit model depicting the correlation between MCD and MVD.
Verification of Mast Cell Density Count
In the above tissue sections, the number of mast cells was counted twice in three optical fields using a magnification x200 (20x objective and 10x occular lens, 0.785mm2 per field) and the mean number of mast cells was estimated. Separate tissue sections from the same tissue blocks were stained with toluidine blue alone. The number of mast cells in three optical fields using a magnification x200 was also counted twice and the mean number of mast cells was estimated to ensure that the same number of mast cells were stained using the simple toluidine blue stainining technique and the counterstaining technique.
Numerical Microvessel Density Assessment
The area of optimal staining for microvessels was marked using a magnification x100. Within the marked area, at x400 (40x objective and 10x occular lens, 0.785mm2 per field) magnification and with a one-field depth from the basement membrane of the epithelium or from the tumor invasive margins or from the tumor nests, three microscopic fields, having the larger number of microvessels, were selected and assesed. Microvessel density was defined as the number of stained microvessels per optical field. Any single stained endothelial cell or endothelial cell cluster, clearly separated from adjacent microvessels, tumor cells, and other connective tissue elements was considered a single, countable microvessel [12]. When different degrees of dysplasia were observed at the same tissue section, microscopic fields around the most severe lesion were selected. Necrotic areas, within the tumor sections were avoided.
Numerical Mast Cell Density Assessment
At the three already selected microscopic fields, having the larger number of microvessels, at x400 magnification and with a one-field depth from the basement membrane of the epithelium, the number of mast cells was counted. Mast cell density was defined as the number of stained mast cells per optical field.
Statistical Analysis
Statistical analysis using the statistical package SPSS 13 (Chicago, Illinois), was performed. In order to test the differences concerning microvessel density (MVD) and mast cell density (MCD) between any two groups of patients the Bonferoni test, after ANOVA, was used at a significance level of p < 0.05. To test if there was a correlation between mast cell density (number of mast cells per optical field) (MCD) and the number of microvessels per optical field (MVD), regression analysis (within the generalized linear models) was performed and the curve fit model was estimated.
RESULTS
Patients and Tissue Characteristics
Out of the 49 patients: 36 were men and 13 women. The average age of these patients was 60.38±14.99 years, with a range of 26-87 years. Primary tumor sites were: the vermilion border, the buccal mucosa, the tongue, the floor of the mouth and other sites within the oral cavity (Tables 1,2).
Correlation of MVD and MCD with Patients’ Characteristics and Tissue Histopathological Differentiation
| Histological Diagnosis | Number of Biopsies | Microvessel Density (Mean ± SD) | Mast Cell Density (Mean ± SD) |
|---|---|---|---|
| Normal | 5 | 14.08±6.08 | 1.32±1.02 |
| Leukoplakia without dysplasia | 7 | 18.18±2.56 | 4.55±3.26 |
| Leukoplakia with dysplasia | 12 | 21.79±6.20 | 7.11±3.20 |
| OSCC | 30 | 24.78± 8.1 | 9.33±6.26 |
| Grade of Dysplasia | |||
| Mild | 4 | 19.92±5.81 | 7.38± 5.15 |
| Moderate | 4 | 19.43±4.16 | 6.86±2.07 |
| Severe | 4 | 22.38±3.57 | 6.15± 3.05 |
| Total | 54 | ||
Correlation of MVD and MCD with Patients’ Characteristics and Tissue Histopathological Differentiation
| Histological Diagnosis | Lesion Site | ||||
|---|---|---|---|---|---|
| Vermilion Border | Buccal Mucosa | Tongue | Floor of the Mouth | Other Sites | |
| NOM | 2 | 1 | 1 | 1 | 0 |
| Leukoplakia without dysplasia | 2 | 2 | 2 | 1 | 0 |
| Leukoplakia with dysplasia | 2 | 4 | 4 | 2 | 0 |
| OSCC | 8 | 7 | 7 | 5 | 3 |
| Grade of Dysplasia | |||||
| Mild | 1 | 1 | 2 | 0 | 0 |
| Moderate | 1 | 2 | 0 | 1 | 0 |
| Severe | 0 | 1 | 2 | 1 | 0 |
Numerical Microvessel Density
In normal oral epithelium, leukoplakia without dysplasia and leukoplakia with dysplasia lesions, microvessels are mainly located just underneath the epithelium and were stained as brown spots, lines or lumens. MVD was counted in every tissue. In normal oral tissues the mean MVD was 14.08, in leukoplakia without dysplasia 18.18, in leukoplakia with dysplasia 21.79 and in OSCC 24.78. The number of microvessels was found to be increased significantly between: Normal oral mucosa and leukoplakia with mild (p=0.022), and severe dysplasia (p=0.04) and also between normal oral mucosa and OSCC (p=0.000). The number of microvessels was also found to be increased significantly between: leukoplakia without dysplasia (p=0.042), leukoplakia with mild (p=0.033), moderate (p=0.010), severe dysplasia (p=0.04) and OSCC, but it was not significantly increased between leukoplakia with severe dysplasia and OSCC (p=0.262) (Table 1, Figs. 1,2,3,4). No correlation was found between the microvessel density and the age of the patients (mean= 22.8 and p=0.30) or the site of the primary tumor (mean=23 and p=0.247).
Numerical Mast Cell Density
Toluidine blue staining revealed mast cells as large, purple, oval and highly granulated cells (Fig. 5). Mast cells were observed at the lamina propria highly populating areas around the tumor margins. Mast cells were also observed at the tumor periphery and at the stem of the lesion when this existed. Increased numbers of mast cells were also found in areas of high vascularization (namely the ‘hot spots’). MCD was counted in every tissue. In normal oral tissues the mean MCD was 1.32, in leukoplakia without dysplasia 4.55, in leukoplakia with dysplasia 7.11 and in OSCC 9.33. The number of mast cells was found to be increased significantly between: normal oral mucosa and leukoplakia with mild (p=0.02), moderate (p=0.011) and severe dysplasia (p=0.029) and also normal oral mucosa and OSCC (p=0.000). The number of mast cells was also found to be increased significantly between: leukoplakia with severe dysplasia and OSCC (p=0.05) (Table 1 and Figs. 1,2,3,4). No correlation was found between the microvessel density and the age of the patients (mean= 22.8 and p=0.30) or the site of the primary tumor (mean=23 and p=0.247).
Correlation Between MCD and MVD
The number of microvessels was found to depend significantly on the number of mast cells (p=0.000) in a percent 28.3%, following the power curve fit model (Fig. 6). The MVD density can then be estimated by the equation: MVD=12.160 x Mast cell density 0.283. According to this power curve fit model, when the density of mast cells per optical field reached the number of 8, no further increase in the density of microvessels related to the density of mast cells was observed.
DISCUSSION
Solid tumors, in order to outgrow the size of 2 mm3, demand for oxygen supply [23], a fact that makes necessary the formation of new microvasculature [5]. The process of angiogenesis is the outcome of an imbalance between positive and negative angiogenic factors produced by both tumor and host cells. Among the host cells, which produce and release in a considerable quantity pro-angiogenic and angiogenic factors are mast cells [24]. Mast cells are bone marrow derived, highly granulated cells, which are an important source of several proangiogenic and angiogenic factors, such as histamin and heparin [25-27], chymase [28], bFGF (FGF-2) [29, 30], VEGF [31].
TGF-beta [32] and others. Some mast cells can release and then rapidly synthesize VEGF [33, 34]. Within the oral cavity, the transformation rate of non dysplastic leukoplakia lesions to oral squamous cell carcinoma is estimated up to 0.4-2 %, whereas the transformation rate of dysplastic leukoplakia lesions up to 9-11% [35]. The literature about the degree of vascularity and its correlation with mast cells in premalignant and malignant oral lesions is conflicting [36-38]. Most of the reports though, measure mast cells and microvessels in different optical fields making their correlation not warranted [39]. Measuring mast cells and microvessels together, at the same optical field seems a more appropriate approach. In the current study therefore we adopted the counterstaing technique in order to comply with this restrictment. Two other reports [13, 15] in the literature also make an attempt towards this idea. In the current study, in order to measure angiogenic activity, a pan-endothelial marker (CD34) was used to mark microvasculature , and a method by Tae et al. [40] rather than the commonly used indirect method of Weidner [41] was adopted in order to count the number of microvessels. Since connective tissue is located just underneath the epithelium it is easier to focus on microvessels, at a x400 magnification, around the main lesion rather than deep in the connective tissue where diffusion of the chromogen is often observed. In the present study, the density of microvessels (MVD) did increase significantly between normal oral mucosa and oral leukoplakia without dysplasia, oral leukoplakia with mild, moderate or severe dysplasia, and oral squamous cell carcinoma. Moreover, the number of microvessels was found to increase significantly between oral leukoplakia and oral squamous cell carcinoma. Therefore an angiogenic switch seems to be turned on, in these early stages of epithelial malignant transformation. This finding comes into accordance with other reports in the literature [42, 43], stressing the need for thorough research on the possible genotypic and phenotypic alterations resulting in this increased early angiogenic activity and on the role that this early angiogenic activity may play in the mechanisms of epithelial malignant transformation. Moreover, in view of these results, a potentially different therapeutic approach during the progression from normal oral mucosa through leukoplakic lesions with various degrees of dysplasia to OSCC, could be discussed. At the same time, the density of mast cells (MCD) was also found to increase between normal oral mucosa and oral leukoplakia without dysplasia, oral leukoplakia with mild, moderate or severe dysplasia, and oral squamous cell carcinoma elucidating a possible role of these cells during the progression from normal oral tissue to oral squamous cells carcinoma. Several reports on a number of malignancies support the above findings [13, 15, 39] whereas one article so far, concerning oral squamous cell carcinoma, reports the opposite idea [17]. In the current study, the counterstaining technique enabled the simultaneous observation of microvessels and mast cells at the same optical field. Mast cells were found to highly populate areas of dense vascularization (namely the ‘hot spots’) and especially around the tumor margins. It is also known that mast cells accumulate near sites of new capillary formation whereas direct association of MCs with vascular tube formation was clearly shown in vitro [44]. The number of microvessels was found to correlate significantly with the number of mast cells (p=0.000) in a percent 28.3%, following the power curve fit model. This correlation is depicted by a certain equation: MVD=12.160 x MCD 0.283. This finding come into agreement with the results from previous studies in oral squamous cell carcinoma 14], laryngeal squamous cell carcinoma [39] and other malignant tumours such as, squamous cell carcinoma of the oesophagus and lungs [45] and carcinoma of the uterine cervix [46]. According to this power curve fit model, when MCD per optical field reached the number of 8, no further increase in the number of microvessels related to the number of mast cells was observed. In the current study the mean number of mast cells in leukoplakia with moderate dysplasia was found to be 8.47 which means that theoretically above the point where leukoplakia with dysplasia progresses to OSCC, no further increase in the number of microvessels related to the number of mast cells is present. The correlation of MVD and MCD seems, according to our findings, to be limited only in the early stages of tumorigenesis (during the transition from normal oral tissue through leukoplakic lesions with various degrees of dysplasia to OSCC) before the full transformation of dysplastic lesions to OSCC, giving place to other angiogenic stimuli or factors afterwards. The above findings could come up as a result of the increase in the number of microvessels allowing the infiltration with more mast cells. On the other hand, this direct correlation of MCD to MVD may be due to the proangiogenic and angiogenic activity of mast cells which has been proposed by previous studies [47, 48]. Several factors secreted by mast cells, such histamine [25], heparin [26], VEGF [31], bFGF [29, 30], and tryptase [27], have been shown to exert an activity on the migration and/or proliferation of endothelial cells. The number of stained mast cells depends on the staining technique (toluidine blue, saphranin O, tryptase immunostaining, etc.), with the tryptase immunostaining appearing to be the most specific for human mast cells [49] and the solution used for the tissue-fixing, with Carnoy’s solution revealing more mast cells than formalin [50, 51]. Moreover, there is no ideal method or endothelial cell marker, which specifically enumerates active or even all microvessel endothelial cells [12, 40, 41, 52]. It is in fact difficult to measure the number of microvessels and probably even mast cells in tissues [24]. In the present study the counterstaining technique was preferred in order to exert the possible angiogenic activity of mast cells at the same optical field with microvessels. More research with various different methods is needed to establish the above correlations and interactions.
CONCLUSIONS
According to the findings of the present study, during the progression from normal oral tissues through leukoplakia with various degrees of dysplasia to OSCC.
- MVD and MCD seem to be significantly increased.
- MCD seems to be significantly correlated to MVD.
The authors would like to thank Mrs Penelope Anastasiadou for her technical assistance.


