All published articles of this journal are available on ScienceDirect.
The Effect of Infection SARS-COV-2 on the Condition of the Salivary Glands – A Retrospective Study
Abstract
Introduction
SARS-CoV-2 promotes the occurrence of short and long-term changes in the oral cavity. This study aimed to present the effect of SARS-CoV-2 on the salivary glands.
Methods
The study group included 138 patients with mild COVID-19 who had various pathologies in the salivary glands. All these patients were treated with antiviral and moisturising agents; some received antibiotics, substances supporting saliva secretion, and saliva substitutes, and some had photobiomodulation and massage of salivary glands.
Results
In the studied group, statistical significance (α≤0.05) was found for xerostomia, ectasia, and sialadenitis, and other changes were statistically insignificant (α>0.05). Severe dryness was observed in 10% of patients, moderate in 28.33%, and mild in 61.66%. Ectasia was found in 29.71% of cases, sialadenitis in 10%, Sjogren's syndrome in 6.5%, neoplasm in 3.62%, and recurrent sialadenitis was found in 6.5%.
Discussion
The conducted study indicates that changes located in the salivary glands after SARS-CoV-2 infection are characterized by clinical diversity, occur more often in women, and may be recurrent. Salivary disorders in patients with and after COVID-19 are they impair here quality of oral health and affect the sense of taste, and cause difficulties in eating food. and increase the risk of mental disorders.
Conclusion
The diseases of the large salivary glands are a significant problem during COVID-19. In the absence of pain and systemic symptoms, observation should be carried out for 4 weeks and wait for spontaneous disappearance. With the simultaneous occurrence of various pathological changes, the patient should be referred for specialist treatment.
1. INTRODUCTION
SARS-CoV-2 promotes the occurrence of short and long-term changes in the oral cavity. Early changes may precede the systemic changes by up to 4 days or occur simultaneously and lead to changes in the flow and composition of saliva, resulting in taste disturbances [1]. Whereas long-term symptoms emerging up to 12 weeks after the general symptoms and last at least two months and cannot be explained by an alternative diagnosis, are called Post-COVID Syndrome (PCS) [2-5]. Some authors believe that PCS can be diagnosed when symptoms last longer than 12 weeks [6]. However, there is still no clear position regarding the terminology and classification of PCS [7]. It has been hypothesized that persistent symptoms after COVID-19, also referred to as Post-COVID Condition (PCC), may be related to the long-term persistence of the virus, abnormal immune response, and/or organ damage [8]. It is also uncertain whether changes located in the oral cavity are the result of the virus or are secondary [9-12]. Based on research, it was found that the oral cavity is the portal and reservoir of the virus [13].
Based on the literature, it can be stated that the symptoms of COVID-19 in the oral cavity include: pain, xerostomia, aphthous-like lesions, erosions, ulcerations, changes in mucosal color, vascular lesions, vesiculobullous lesions, periodontal necrosis, candidiasis, tongue lesions ranging from strawberry tongue through atrophic, nodular lesions to geographic tongue, exacerbation of autoimmune diseases, Kawasaki-like disease, Erythema Multiforme-like, atypical Sweet syndrome, Melkerson-Rosenthal syndrome, aphthous stomatitis, angular cheilitis, herpetic lesions, varicella-zoster virus infection (VZV), drug rash, vasculitis, thrombi, perivascular lymphocytic infiltration, focal capillary thrombosis, hemorrhages [14-17].
COVID-19 has also affected dentistry and dental treatments. Based on a survey study . A similar number stated that emergency dental procedures should be performed in the event of conducted in Nepal, nearly a quarter of dentists were not working in dental offices. A similar number stated that emergency dental procedures should be performed in the event of a COVID-19 infection. Patients received dental treatments from only 10% of dentists during the lockdown, and 53% of dentists participated in online training. 70% of dentists experienced financial difficulties due to a lack of remuneration. 86% of respondents indicated that dental procedures should be performed regularly despite the pandemic [18].
Pathological changes in the salivary glands have accompanied SARS-CoV-2 infection since the beginning of the pandemic [1]. Xerostomia is recognized by the WHO as one of the symptoms of COVID-19. Epithelial cells of the salivary glands can become infected and be the source of the virus in saliva, which is crucial for the development of effective strategies for diagnosis, prevention, and therapy [19-21]. Based on research, it was found that the salivary glands may be one of the transmission routes of the SARS-CoV-2 virus, which, after replicating in the oral cavity, can spread with saliva [20]. Angiotensin-converting enzyme receptors 2 (ACE2) and Transmembrane serine protease 2 (TMPRSS2) present in the acinar cells and ducts of the large salivary glands are expressed, which promotes the entry of the virus and its replication [20, 21].
However, information regarding xerostomia related to COVID-19 should be interpreted carefully, because many causes contribute to the appearance of dry mouth. SARS-CoV-2 infection induces cytokine release syndrome and thrombocytopenia, which may be accompanied by inflammation, fibrosis, hyperplasia, metaplasia, precancerous conditions of the salivary glands, or autoimmune diseases (e.g., Sjogren's syndrome). It was found that the above-mentioned pathologies are most often located in the parotid and submandibular glands [22-28].
The available literature also contains information that changes in the salivary glands may result from post-vaccination complications, the so-called adverse event reaction [29].
It should be noted that salivary flow is influenced by many local factors (e.g., oral hygiene, decreased cortisol or amylase levels, ult), radiotherapy) and systemic factors, including medications (e.g., psychotropic, antidepressants, antihistamines, diuretics, chemotherapy), dehydration, dietary composition, psychological factors (stress, anxiety, depression), various systemic diseases (e.g., diabetes, HIV, HCV, hypertension, Alzheimer's disease), and disorders of the central and peripheral nervous systems [30, 31]. The amount and quality of saliva also change with age, but this relationship is not directly proportional, as salivary flow is influenced by factors such as local and general health, medications, and preferred lifestyle [32].
The study aimed to present various changes located in the salivary glands after SARS-CoV-2 infection and to assess their disappearance depending on the therapy used or its lack.
2. MATERIALS AND METHODS
The study followed the Sex and Gender Equity in Re search guidelines (SAGER) to ensure appropriate inclusion and reporting of sex-related data (Appendix). The research was planned and conducted using the STROBE checklist. The Warsaw Medical University’s Bioethical Commission has approved the range of the retrospective study (Commission statement AKBE/151/ 2024).
The data were obtained from the medical records of 1,256 patients treated at our dental clinic from 2020-2023. The minimum sample size calculated using the Raosoft® calculator was 135 persons with a 5% margin of error and a 95% confidence level. The sample power for the Type II error probability calculated using the G* Power 3.1 application was 0.8532830. The search was based on SARS-CoV-2, RT-PCR, changes in the salivary glands, xerostomia, therapeutic methods, and supportive therapy. Data analysis allowed us to select 138 patients (including 86 women and 52 men) aged 20-40 after a mild COVID-19 infection who had various pathologies in the salivary glands. The patients were previously healthy and had no problems with salivation, allergies, or addictions. The exclusion criteria were comorbidities, pregnancy, addictions, and stimulants. Patients gave written consent to provide research documentation.
Patients were assigned a random ID number that did not allow them to be identified in the clinic's records. Patient records were copied to a password-protected AES 256 encryption external SSD, to which only researchers had access. After finishing the data analysis, the documentation was deleted from the SSD.
The examination of each patient was performed during the first visit, periodically (Table 1), and when reporting dental problems.
In the case of the diagnosis of xerostomia, the severity of dryness was assessed on a Challacombe scale of 0-10 (0 - no dryness, 10 - severe case of dryness). The test was repeated after two weeks.
All treated patients were treated with an antiviral agent, Heviran 800 mg (Polpharma), and a moisturizing agent with a biocidal effect, Fomukal (Vipharm). Patients with sialadenitis or ectasia were additionally treated with an antibiotic, Clindamycin 600 (MIP Pharma). The patients who had enlarged salivary glands and swelling were treated with photobiomodulation (PBM) and gentle massage. Patients with xerostomia were additionally recommended to use substances supporting saliva secretion (e.g., drinking water with lemon, chewing sugar-free gum, or eating sour candies) and saliva substitutes - Xerostemin aerosol (Atos).
All patients were recommended increased hygiene, including the use of alcohol-free mouthwash.
Patients were informed to avoid alcohol and products containing caffeine.
If the swelling persisted for more than 4 weeks, the patient was referred for laboratory tests and on ultrasound (US) or to a specialist centre.
The documentation analysis enabled us to determine the time of remission of the pathology, depending on whether therapy was used or not.
Descriptive statistics functions in MS Excel 365 were used for statistical analysis of quantitative data. The outcomes included the total number of subjects by gender (N), the number of valid observations (n), the arithmetic mean (x̄), and the percentage of patients with specific changes in salivary glands, including xerostomia and their duration. The level of statistical significance was adopted as α=0.05 and p-value ≤ 0.05, which allowed us to reject the hypothesis about the lack of association between COVID-19 and salivary gland pathologies.
3. RESULTS
The results of this retrospective study were presented in 2 tables and 3 figures. Changes in the major salivary glands in women and men, and their duration, were presented in Table 2. The time of changes disappearance after treatment or without therapy was presented in Table 3. The figures present sample photos of the xerostomia, the left parotid gland inflammation, and the neoplasm.
Based on the analysis of medical records of the study population of 1,256 patients, it was found that various changes affecting the salivary glands occurred in 11% of people, including 6.8% of women and 4.2% of men.
71 women experienced three coexisting pathologies: xerostomia, ectasia, and sialadenitis. Only one pathology, xerostomia, occurred in 13 men.
Unilateral changes in the salivary glands occurred in 38 patients (27.53% of cases) and were usually accompanied by xerostomia.
Xerostomia (Fig. 1) was found in 60 people (47 women and 13 men). In 42 patients, it appeared 3-4 days before the systemic symptoms of SARS-CoV-2 infection, and in 18, it was observed approximately three months after the appearance of systemic symptoms. In 37 patients, mild dryness (VAS = 1–3), in 17 moderate dryness (VAS = 4–7), and 6 severe dryness (VAS = 8–10) was observed. In 7 people (5% of cases), dryness persisted intermittently for 36 months.
| Medical Examination | Periodicity | Checklist |
|---|---|---|
| Subjective and objective examination | First visit, one and two weeks after the treatment was started. In the first year of observation every month, in subsequent years every 2 months or when a problem occurs. | Oral condition, date of appearance of the first and subsequent symptoms, complications, current complaints, systemic diseases, medications taken, and addictions. |
| Laboratory tests | First visit, next every 6 months. | In case of deviations from the norm, referral to a specialist. |
| Thorax X-ray or CT scan | First visit, next every 12 months. | In smoking and COPDA patients. |
| OPG/OPTB | First visit, next every 6 months. | Assessment of the condition of stomatognathic system. |
| USC | In case of swelling of the salivary glands lasting 4 weeks. | In case of deviations from the norm, referral to a specialist. |
| CODSD | First visit and after 2 weeks. | The severity of dryness on the Challacombe scale. |
OPG/OPTB – Orthopantomography.
USC – Ultrasonography.
CODSD – Clinical Oral Dryness Score.
| No. | Type of Pathological Change in the Major Salivary Glands | Gender | Duration (months) | |
|---|---|---|---|---|
| Women | Men | |||
| 1. | Xerostomia | 47 | 13 | 3 to 36 |
| 2. | Ectasia | 29 | 12 | 3 to 36 |
| 3. | Sialadenitis | 8 | 6 | 3 to 9 |
| 4. | Recurrent Sialadenitis | 6 | 3 | Up to 36 |
| 5. | Sjögren's syndrome | 8 | 1 | 3 to 36 |
| 6. | Neoplasic metaplasia | 3 | 2 | Diagnosed after 12 months of PCS |
| No. | The Salivary Glands Pathology |
Number of Valid Patients | Duration (days) | Statistical Significance | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treated (n) |
Not Treated (n) |
Treated | Not Treated | - | ||||||
| Min. | Max. | Mean x̄ |
Min. | Max. | Mean x̄ |
α | ||||
| 1. | Xerostomia | 36 | 24 | 7 | 547 | 10,5 | 14 | 1080 | 547 | <0,05 |
| 2. | Ectasia | 12 | 29 | 5 | 543,5 | 9,5 | 7 | 1080 | 543,5 | <0,05 |
| 3. | Sialadenitis | 11 | 3 | 7 | 185 | 17,5 | 10 | 360 | 185 | =0,05 |
| 4. | Recurrent sialadenitis | 7 | 2 | 28 | 720 | 74 | 180 | 1080 | 720 | >0,05 |
| 5. | Sjögren's syndrome | 9 | - | 60 | - | 570 | - | - | - | >0,05 |
| 6. | Neoplasm | 5 | - | 180 | - | 630 | - | - | - | >0,05 |
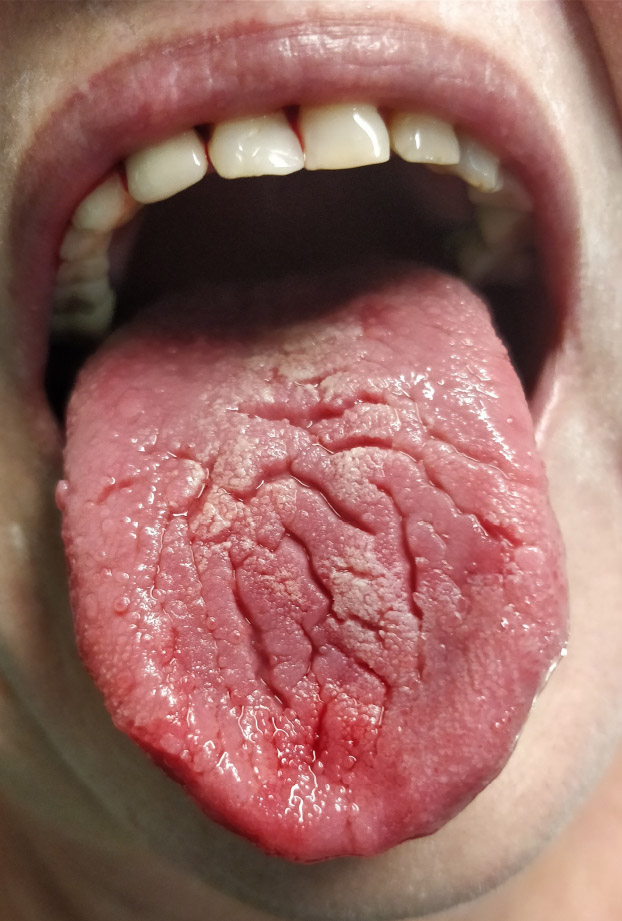
Sample photo of xerostomia.
Salivary gland ectasia was found in 41 patients (29 women and 12 men). At 22 people (53.65%), it was found in the parotid glands, at 13 people (31.7%), it was found in the sublingual glands, and at 6 people (14.63%), it was found in the submandibular glands.
Sialadenitis (Fig. 2) was found in 14 patients (8 women and 6 men), and recurrent sialadenitis in 6 women and 3 men.
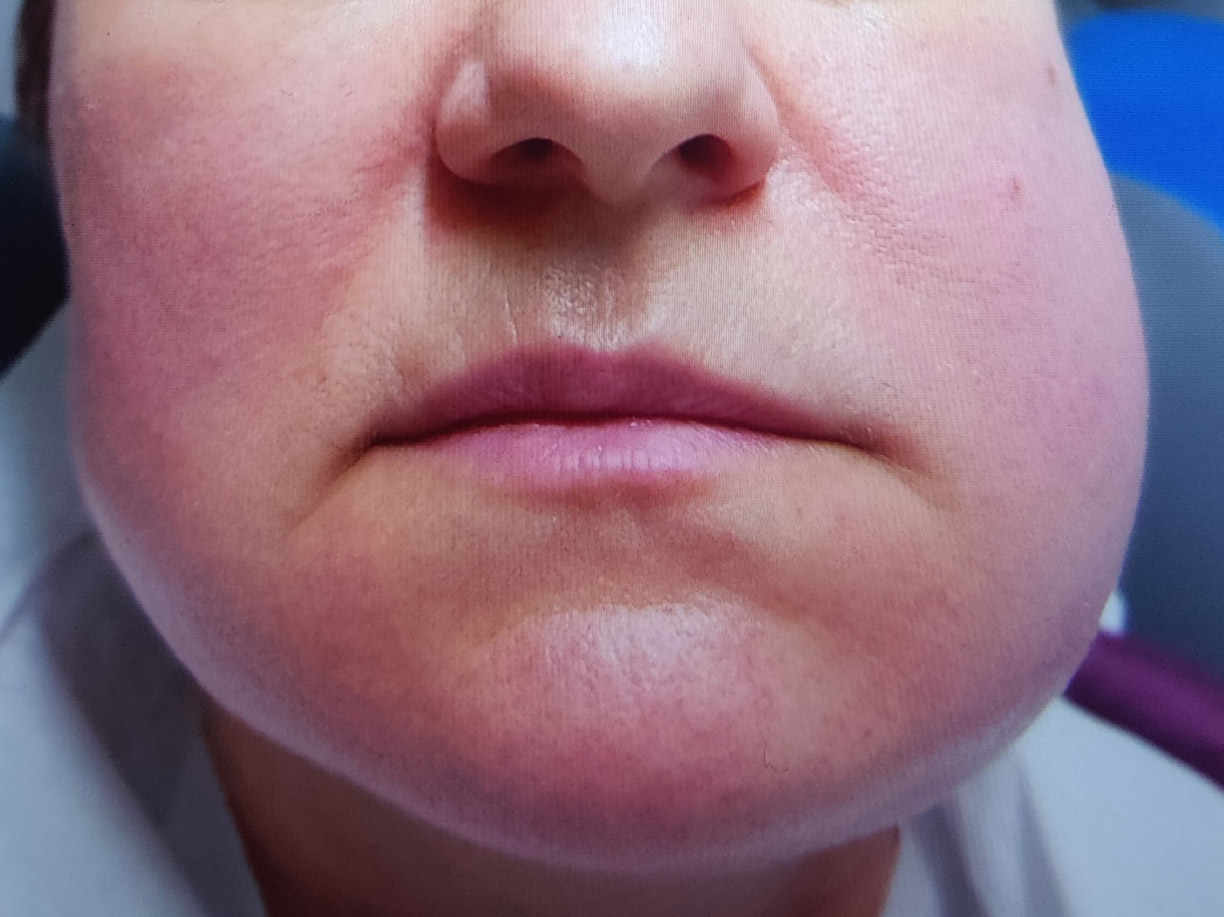
Sample photo of the left parotid gland inflammation.
Sjögren's syndrome was diagnosed in 8 women and 1 man.
Neoplasm was found in 3 women and 2 men (Fig. 3). In the interview, the patients reported that they were previously generally healthy, did not take medications or dietary supplements, and did not smoke or drink alcohol. In the acute phase of SARS-CoV-2, they had a unilateral enlargement of the parotid glands and xerostomia for 7 days, as well as loss of smell, taste, and flu-like symptoms. These patients are under the care of an oncology hospital.
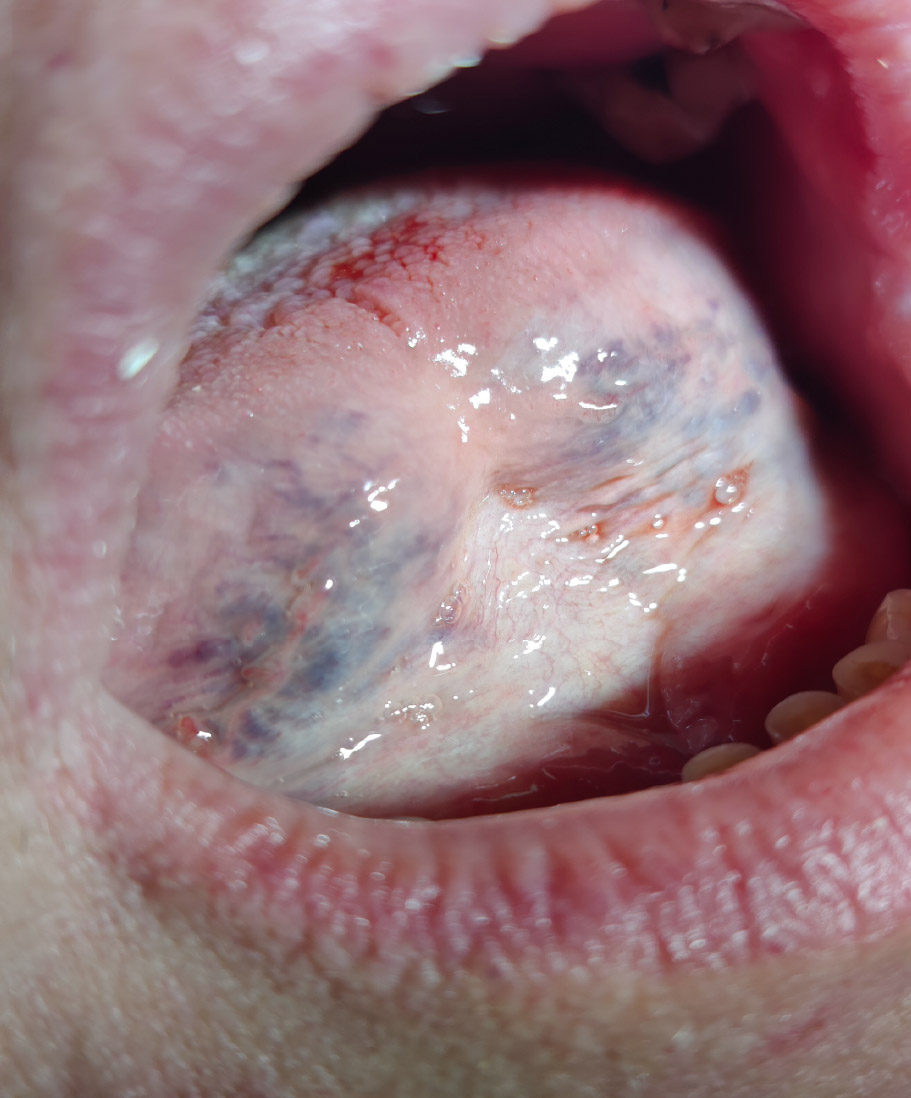
Sample photo of neoplasm.
In 26 patients (18.84% of cases), salivary gland swelling resolved spontaneously within 7 days.
In the studied group of patient’s statistical significance (α≤0.05) was found for xerostomia, ectasia, and sialadenitis, and other changes were statistically insignificant (α>0.05).
4. DISCUSSION
Given the importance of salivary glands and xerostomia for safety and quality of life, it is essential to address the long-term frequency and persistence of COVID-19-related oral complications, which remain unclear and insufficiently researched. The conducted study allows us to state that changes located in the salivary glands after SARS-CoV-2 infection are characterized by clinical diversity, occur more often in women, and may be recurrent.
Xerostomia occurred in 43.47% of cases - in 70% it preceded systemic symptoms, and in 30% it appeared after a few weeks. Severe dryness was observed in 10% of patients, moderate in 28.33%, and mild in 61.66%. In the case of severe dryness, patients additionally reported dysphagia, frothy saliva, and dry lips. Disturbed salivary secretion lasted from 7 to 1080 days.
The prevalence of xerostomia due to COVID-19 in various studies conducted in different countries ranges from 40 to 60% [34-37]. The severity of xerostomia is associated with the severity of infection [38, 39]. In the study, the highest prevalence was observed in patients aged 68-77 years (33.3%), followed by patients aged 78-83 years (22.27%). A higher prevalence of xerostomia was found among menopausal women (17.97% vs. 10.47%). A statistically significant association was found between the use of antidepressants (p < 0.001), antihypertensive drugs (p < 0.001), and antihistamines (p = 0.045) and xerostomia [40].
In most cases, it occurs before other symptoms of the disease, and its severity often decreases after 15 days [5]. The etiological factors of xerostomia include more than 400 medications (antiparkinsonian, antipsychotic, antidepressant, diuretic, opioids, cytotoxic, and antihypertensive), systemic diseases (endocrine/autoimmune/ infectious/ granulomatous), and environmental factors (radiation/lifestyle) [1, 13, 40-43]. We need to obtain information about the psychotropic drugs we are taking in our medical history. As we know, more and more patients are being treated for psychological problems. Biochemically, xerostomia may be associated with electrolyte imbalance (Na+, K+, Cl-, or Ca2+), enzymatic changes (e.g., increased amylase concentration), changes in protein composition (e.g., decreased mucin concentration), impaired function of G-Protein-Coupled Receptors (GPCRs) found on salivary cells (saliva-producing cells), and reduced ability of alveolar cells to produce/secrete saliva due to impaired movement of the Aquaporin 5 (AQP5) water channel [44, 45].
It was found that earlier variants of COVID-19 (Alpha, Beta, Gamma, and Delta) promoted the appearance of xerostomia more often than the Omicron variant [28].
The appearance of dry mouth before the onset of other symptoms of SARS-CoV-2 supports early diagnosis of the infection and allows for the implementation of treatment, which, according to some authors, may limit the transmission of the virus [1, 12].
Another confirmed pathology was ectasia, which was found in 29.71% of cases and lasted from 5 to 1080 days. After the therapy, the swelling usually disappeared after 14 days. However, when patients did not consent to treatment, the swelling persisted for up to 36 months.
If ectasia occurs in the initial phase of infection by SARS-CoV-2, the concentration of C-reactive protein and LDH changes. The levels of the above-mentioned biomarkers indicate the severity of the infection [20, 46, 47].
Salivary gland ectasia often precedes the sialadenitis [19, 23, 24, 42]. However, swelling of the salivary glands due to viral infection (viral sialadenitis) has not been fully documented in the case of COVID-19. Publications on this subject are limited to case reports [22-27, 46, 47].
The medical records of 10% of the patients described sialadenitis. Parotid and sublingual gland inflammation associated with COVID-19 has been reported since the beginning of the pandemic [24, 25].
Within the salivary glands, SARS-CoV-2 may lead to an inflammatory reaction and various pathological changes, including autoimmune ones [27, 46, 48].
Sjögren's syndrome was diagnosed in 6.5% of patients. These patients were referred for rheumatological treatment. According to the literature, patients with SARS-CoV-2 who additionally have autoimmune salivary gland diseases may develop immunoproliferative diseases, mainly non-Hodgkin lymphoma [27, 49].
The proliferation of B lymphocytes and their continuous activation because of autoimmune processes increases the incidence of lymphomas and multiple myeloma 6-18 times [49-52].
Impaired production and secretion of histatin into saliva in patients with COVID-19 due to the destruction of the salivary glands predisposes them to long-term opportunistic infections and inflammation of the mucous membranes, which may result in neoplasms [49-52].
In the records of 3.62% of patients, neoplasm changes were found. These patients were referred for further oncology diagnostics.
Uncontrolled autoreactive mechanisms may occur in patients after vaccination against SARS-CoV-2, which favours the development of autoimmunity in people with a strong genetic predisposition. Therefore, autoimmune diseases have been diagnosed in some people after vaccinations [26, 29, 52-56].
Differential diagnoses of pathological conditions of the salivary glands in PCS include diseases of the parotid lymph nodes (tuberculosis, cat scratch disease), hemangiomas, tumours (lymphomas, sarcomas), enlargement against the background of other diseases (diabetes, anorexia), lymphatic malformations, or taking medicaments that reduce salivary secretion [2, 4, 19, 22, 46-48]. The pandemic lockdown has led to the development of psychological problems [57, 58]. The consequences of stressful working conditions and social isolation have increased the incidence of Temporomandibular Disorders [19]. The psychological impact of this pandemic has been profound, with studies demonstrating a high prevalence of anxiety and depression among patients and healthcare workers [18, 58]. Studies conducted across different countries have demonstrated significant anxiety levels among healthcare workers with an urgent need for interventions. Difficult access to a doctor resulted in a lack of diagnosis and early treatment, which led to complications. It should also be noted that the dental team is at increased risk of SARS-Cov-2 infection during procedures. Literature reports indicate the need for risk assessment for both patients and office staff, strict use of personal protective equipment and disinfectants, use of disposable instruments, limiting aerosol generation (e.g., putting off invasive procedures), and proper decontamination [58].
5. STUDY LIMITATION
The study's design is limited, as it involves analyzing the medical records of patients in a specific age range who were treated only at our clinic. The selection of the study group from among previously healthy patients also limits the scope of the study. It would be advisable to continue in future similar studies, but involve patients in different age groups, burdened with various diseases, and treated with different methods.
CONCLUSION
The results of this study indicate that diseases of the large salivary glands are a significant problem in PCS. When diagnosing a patient with a history of coronavirus infection, a thorough medical history, physical examination, and additional tests should always be performed, along with careful differentiation. Many symptoms of systemic, immunological, or viral diseases manifest as changes in the salivary glands. Due to the recurrence of post-COVID changes, patients should be monitored regularly. Salivary disorders in patients with LC are not life-threatening, but they impair the quality of life related to oral health and nutrition, and increase the risk of depression and suicidal thoughts. In the case of pathologies without pain and systemic symptoms, observation should be carried out for 4 weeks and then wait for their spontaneous resolution. If various pathological changes occur simultaneously, the patient should be referred for specialist treatment.
AUTHORS’ CONTRIBUTIONS
The authors confirm contribution to the paper as follows: B.R.: Study conception and design; B.R., J.R., L.W.: Data collection, drafting the manuscript; B.R., L.W.: Analysis and interpretation of results; L.W.: Supervision. All authors approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| PCS | = Post-COVID Syndrome |
| PCC | = Post-COVID Condition |
| VZV | = Varicella-Zoster Virus |
| WHO | = World Health Organization |
| ACE2. | = Angiotensin-converting enzyme receptors 2 |
| TMPRSS2 | = Transmembrane serine protease 2 |
| BMS | = Burning Mouth Syndrome |
| HIV | = Human Immunodeficiency Virus |
| HCV | = Hepatitis C virus |
| RT-PCR | = Reverse-Transcription Polymerase Chain Reaction |
| AES 256 | = Advanced Encryption Standard |
| SSD | = Solid-State Drive |
| PBM | = Photobiomodulation |
| US | = Ultrasound |
| GPCRs | = G-Protein-Coupled Receptors |
| AQP5 | = Aquaporin 5 |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved (Commission statement AKBE/151/2024) by the Warsaw Medical University Bioethics Commission (Poland).
HUMAN AND ANIMAL RIGHTS
The retrospective study was carried out according to the ethical standards of the local bioethics commission. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or research committee and with the 1975 Declaration of Helsinki, as revised in 2013.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article will be available from the first author [J.R] upon reasonable request.
FUNDING
The study was financed within the statutory financial resources WLS7/N/24 of the Medical University of Warsaw, Poland.
ACKNOWLEDGEMENTS
Declared none.
APPENDIX
| Section/ Topic | Item Number | Checklist Item | Reported on Page Number |
|---|---|---|---|
| General | 1 | The terms sex/gender used appropriately | 2,4,5,6, Table 2 |
| Title | 2 | Title specifies the sex/gender of participants if only one included | X |
| Abstract | 3a | Abstract specifies the sex/gender of participants if only one included | X |
| 3b | Study population described with gender/sex breakdown* | 2 | |
| Introduction | 4a | If relevant, previous studies that show presence or lack of sex/gender differences or similarities are cited | X |
| 4b | Mention of whether sex/gender might be an important variant and if differences might be expected | X | |
| 4c | The demographics of the study population with regard to sex/gender (eg, disease prevalence among male/ female study participants) are outlined* |
X | |
| Methods | 5a | Method of definition of sex/gender (eg, self-report, genetic testing) | 4 |
| 5b | Description of how sex/gender was considered in the design, whether authors ensured adequate representation of male and female study participants, justification of the reasons for any exclusion of male or female participants, or explanation if not considered. Justification of other sex/gender-specific interventions of study designs (i.e, mandating contraception for women).* Explicit reporting of the scientific rationale for contraception requirements and exclusions for pregnancy and lactation should be required* | 4 | |
| Results | 6a | Study population description with complete gender/sex breakdown for all categories considered* | 5,6 |
| 6b | Where appropriate, data presented disaggregated by sex/gender, and sex/gender differences and similarities are described | 5,6 Table 2 |
|
| 6c | Sex- and gender-based analyses reported regardless of outcome (in main paper if pre-specified; otherwise in appendix)* | 5,6 | |
| 6d | For clinical trials, adverse event data disaggregated by sex/gender (in main paper if pre-specified; otherwise in appendix)* | X | |
| 6e | Patient-reported outcome data disaggregated by sex/ gender (in main paper if pre-specified; otherwise in appendix)* | 6 | |
| 6f | For epidemiological studies, the effects of other exposures on health problems examined for all genders and analysed critically from a gender perspective | X | |
| 6g | Table 1 includes separate rows for male sex/gender, female sex/gender and other categories if collected* | Table 2 | |
| Discussion | 7a | Potential implications of sex/gender on the study results and analyses, including the extent to which the findings can be generalized to all sexes/genders in a population | 6 |
| 7b | If a sex/gender analysis not done, a rationale is given and implications of the lack of such analysis on the interpretation of the results are discussed |
X |
* These points extend beyond the original SAGER table.


