All published articles of this journal are available on ScienceDirect.
Influence of Bioactive Desensitizers on Bonding of Self-adhesive Resin Cement: A SEM/EDX Microanalysis Study
Abstract
Introduction
Bioactive desensitizing agents are increasingly used to alleviate dentin hypersensitivity; however, their impact on the bonding performance of self-adhesive resin cements remains unclear. This in vitro study evaluated the effect of bioactive desensitizers on the bond strength of a self-adhesive resin cement to dentin and examined elemental changes on the dentin surface using Energy-Dispersive X-ray (EDX) spectrometry.
Methods
Dentin surfaces of extracted molars were exposed and treated with a glutaraldehyde-based desensitizer (Dentin Desensitizer; DD), a calcium phosphate-based desensitizer (Teethmate Desensitizer; TM), a hydroxyapatite-based desensitizer (Predicta Bioactive; PB), or fluoride gel (APF Fluoride Gel; FL). The control group received no treatment. Self-adhesive resin cement (Panavia SA Cement Universal) was applied in cylindrical molds. After thermocycling, microshear bond strength was measured, and failure modes were analyzed. Scanning Electron Microscopy (SEM) combined with EDX analysis was performed to assess changes in the dentin surface.
Results
The DD group showed the highest bond strength (11.15 ± 1.32 MPa), followed by PB (5.98 ± 1.79 MPa), the control group (5.08 ± 1.17 MPa), and FL (4.78 ± 0.75 MPa). TM showed the lowest value (4.09 ± 0.52 MPa). ANOVA with post-hoc comparisons confirmed significant differences, with DD outperforming all other groups (p < 0.001). Adhesive failure was the most common, followed by mixed-type failures. EDX results indicated increased mineral and decreased carbon content across all treated groups, most notably in the PB group.
Discussion
Although bioactive desensitizers altered dentin composition, their inconsistent effect on bond strength may be attributed to the distinct microstructural changes each induces.
Conclusion
Clinicians must carefully consider the balance between enhancing patient comfort and preserving the long-term integrity of the restoration.
1. INTRODUCTION
Dentin hypersensitivity is a common issue that can occur after tooth preparation for indirect restorations. During preparation, millions of dentinal tubules are exposed, leading to increased dentin permeability and potential pulpal irritation. Several factors can elevate the temperature within the pulp chamber and exacerbate dentin hypersensitivity, including insufficient water cooling, excessive air pressure, or applying excessive cutting force [1]. Additionally, increased surface area of the preparation, a prolonged provisional treatment phase, and inadequate sealing of temporary restorations can further contribute to hypersensitivity [2, 3]. This presents a clinical challenge, as managing dentin hypersensitivity is essential for patient comfort and the long-term success of restorative treatment.
According to the hydrodynamic theory, external stimuli, whether thermal, chemical, osmotic, or tactile, cause movement of intratubular dentinal fluid in exposed dentin, which activates underlying nerve fibers and triggers a pain response [4]. Emerging evidence suggests that odontoblasts may also contribute to dentin hypersensitivity by sensing stimuli and releasing pain mediators that signal nearby nerves [3, 5]. To alleviate dentin hypersensitivity, many dentists incorporate desensitizing agents into their protocols after tooth preparation for indirect restorations [5]. These agents function either by desensitizing the nerve fibers, reducing their responsiveness to external stimuli, or by occluding the dentinal tubules to prevent fluid movement [5, 6]. Glutaraldehyde/ Hydroxyethyl Methacrylate (HEMA), oxalates, and fluoride compounds are among the most commonly used formulations [3, 5]. Recently, bioactive desensitizers containing nano-hydroxyapatite and bioactive glass have gained attention for their dual function in managing dentin hypersensitivity and promoting remineralization of tooth structure [3, 7]. Clinical studies have shown that nano-hydroxyapatite may offer superior efficacy in reducing dentin hypersensitivity compared to traditional agents [8]. In addition to providing immediate relief, the deposition of Hydroxyapatite (HA) and other minerals by these bioactive desensitizers may enhance dentin resistance to secondary caries around the restorations [9, 10].
Despite these potential advantages, the influence of newly introduced bioactive desensitizers on the bond strength of resin cements to dentin remains insufficiently studied. Previous reports have suggested that certain bioactive agents may improve the bonding interface by creating a more stable and mineralized surface, potentially leading to stronger and durable bonding [11]. However, the efficacy of these agents can vary depending on their composition, application protocols, and interactions with both dentin and the resin cement. Additionally, the composition and adhesion mechanism of the resin cement itself significantly affect bonding performance, as factors such as the acidity, hydrophilicity, and type of functional monomers influence the cement's interaction with dentin [12].
Self-adhesive resin cements simplify the bonding process by combining adhesive and cementing functions into a single product, eliminating the need for separate etching, priming, and bonding steps. While these cements offer convenience and reduced technique sensitivity, concerns remain regarding long-term durability [12]. Furthermore, studies on crown retention when desensitizers are used prior to cementation have shown mixed results, with some reported a reduction in retention, while others found no adverse effects [13, 14].
Therefore, this in vitro study was conducted to explore the influence of bioactive desensitizers on dentin bonding and surface composition, using Energy-Dispersive X-ray (EDX) analysis to quantify elemental changes and evaluate their effects on the bond strength of self-adhesive resin cement. The null hypothesis tested was that there is no significant effect of desensitizing agents on the bond strength of self-adhesive resin cement or on the elemental composition of the dentin surface.
2. MATERIALS AND METHODS
All procedures in this in vitro experimental study were conducted in compliance with relevant laws and institutional guidelines. The study protocol was reviewed and approved by the institution’s Research Ethics Committee (Approval reference number: 88-09-24). Extracted human molars, free of caries and restorations were collected with patients’ informed consent and in accordance with the principles of the Declaration of Helsinki. Four different desensitizing agents were used: a fluoride gel (APF Fluoride Gel, FL; Keystone Industries, Gibbstown, NJ, USA), a glutaraldehyde-based desensitizer (Dentin Desensitizer, DD; Pulpdent, Watertown, MA, USA), a calcium phosphate-based desensitizer (Teethmate Desensitizer, TM; Kuraray Noritake Dental, Tokyo, Japan), and a hydroxyapatite-based desensitizer (Predicta Bioactive Desensitizer, PB; Parkell, Edgewood, NY, USA). The resin cement used was Panavia SA Cement Universal (Kuraray Noritake Dental, Tokyo, Japan). The composition and application method of each material are listed in Table 1.
2.1. Specimen Preparation
Molar teeth were sectioned below the cementoenamel junction using a diamond saw under water cooling (IsoMet Low Speed Precision Cutter; Buehler, Lake Bluff, IL, USA) and mounted in acrylic resin blocks. The occlusal surfaces were ground perpendicular to the long axis of the tooth using a model trimmer (MT 10, Ray Foster, Huntington Beach, CA, USA) until the dentin was exposed. Dentin exposure was verified under a stereomicroscope (Eclipse MA100, Nikon, Tokyo, Japan) at 40× magnification. The dentin surfaces were then polished with 600-grit silicon carbide paper for 60 seconds to produce a standardized smear layer. The specimens were randomly divided into five groups, with eight specimens in each group (n = 8). Group 1 served as the control group and received no desensitizing treatment. In Groups 2 to 5, the respective desensitizing agents were applied to the dentin surfaces according to the manufacturer's guidelines and allowed to react for the recommended time.
After 24 hours of storage in artificial saliva (pH 6.5) at 37 °C, a polyethylene tube with an internal diameter of approximately 1.8 mm and a height of 2 mm was placed on the dentin surface, and the resin cement was injected into each tube. A glass slide was placed over the cement, gently pressed, and then light cured with an LED curing unit (Elipar, 3M ESPE, St. Paul, MN, USA) at 1200 mW/cm2 for 20 seconds from both the top and sides to ensure complete polymerization. Specimens were then stored for 24 hours in 100% relative humidity at 37 °C. Afterwards, the plastic tubes were removed, and the specimens underwent thermocycling for 5000 cycles between 5 °C and 55 °C, with a dwell time of 20 seconds in each bath and a transfer time of 5 seconds between baths (Thermocycler 1100, SD Mechatronik, Feldkirchen- Westerham, Germany) [15].
2.2. Microshear Bond Strength Testing (μSBS) and Failure Mode Analysis
After thermocycling, each specimen was secured in the lower fixed head of a universal testing machine (Instron, Norwood, MA, USA). A 0.14-inch-diameter stainless steel wire was attached to the upper movable head of the testing apparatus and positioned as close as possible to the cement-dentin interface. The microshear bond strength (μSBS) test was conducted at a crosshead speed of 1 mm/min until bond failure occurred. The maximum load was recorded, and μSBS was calculated in MPa by dividing the load (N) by the bonded area (mm2). The specimens were subsequently mounted on aluminum stubs using carbon adhesive tape and examined under a Scanning Electron Microscope (SEM; Quanta 3D 200i, FEI Company, Hillsboro, OR, USA). Failure modes were classified as adhesive, cohesive, or mixed. Adhesive failure refers to failure at the adhesive interface, cohesive failure indicates failure within the adhesive or dentin, and mixed failure represents a combination of adhesive and cohesive failure.
2.3. Energy-dispersive X-ray Spectrometry (EDX)
Elemental distribution on dentin surfaces from each test group was analyzed using EDX under SEM. The SEM was operated at an accelerating voltage of 20 kV, with a resolution of 1 nm, and magnifications of 90x and 250x. Initial EDX analysis provided a qualitative overview of elemental composition across the groups, identifying peaks corresponding to various elements and their relative intensities. Elemental quantification was then performed, and data were recorded as both weight percentages and atomic percentages to assess compositional changes in dentin.
2.4. Statistical Analysis
The μSBS data were expressed as mean values along with 95% Confidence Intervals (CI), Standard Deviation (SD), and the range, which included both the minimum (min.) and maximum (max.) values. To determine whether the data were suitable for parametric analysis, normality was tested using the Shapiro-Wilk test, and homogeneity of variances was assessed with Levene’s test. Both assumptions were satisfied across all groups, allowing for further analysis using a one-way ANOVA, followed by Tukey’s post hoc test for group comparisons with a significance level of p<0.05 (R software version 4.4.0, R Core Team, 2024, Vienna, Austria).
| Material (Abbreviation) Manufacturer |
Composition | Manufacturer Application Instructions |
|---|---|---|
| APF gel (Fl) Keystone Industries, Gibbstown, NJ, USA |
1.23% fluoride, citric acid, phosphoric acid, magnesium aluminum silicate, sodium benzoate, titanium dioxide, water, xylitol | Apply on the surface for 60 s and remove excess |
| Dentin desensitizer (DD) Pulpdent, Watertown, MA, USA |
5% Glutaraldehyde, fluoride, water | Apply to dentin for 20-30 s using a cotton pellet, blot or apply short blast of air to remove excess, but do not dry |
| Teethmate desensitizer (TM) Kuraray Noritake Dental, Tokyo, Japan |
Powder: tetracalcium phosphate (TTCP) and dicalcium phosphate anhydrous (DCPA) Liquid: water |
Mix the powder and water for 30 s to form a slurry, apply with a microapplicator for 15 s, rub for 60 s, rinse off with a water spray |
| Predicta bioactive desensitizer gel (PB) Parkell, Edgewood, NY, USA |
Calcium, phosphate, nanohydroxyapatite | Rinse and dry surface with cotton pellet, apply a coat of the liquid and gently rub it in for 10–20 s. Wipe off the product with a cotton pellet before air blowing |
| Panavia SA Cement Universal Kuraray Noritake Dental, Tokyo, Japan |
Paste A: MDP, Bis-GMA, TEGDMA, dimethacrylate, HEMA, fillers, photoinitiator, peroxide, catalysts, pigments Paste B: dimethacrylate, silane coupling agent, fillers, sodium fluoride, photoinitiator, accelerators, pigments |
Dispense equal amounts of paste A & B, mix for 10 s. Apply and light cure for 10 s |
3. RESULTS
Mean with standard deviation values for μSBS are presented in Fig. (1a). Results showed a significant difference between the test groups (f = 28.28, p < 0.001). The highest μSBS (MPa) was measured in DD (11.15 ± 1.32), followed by PB (5.98 ± 1.79), then the control group (5.08 ± 1.17), and FL (4.78 ± 0.75), while the lowest bond strength was measured in TM (4.09 ± 0.52). Post hoc pairwise comparisons revealed that the DD group had significantly higher bond strength values than the other groups (p < 0.001). No statistically significant differences were detected among the other groups (p > 0.05).
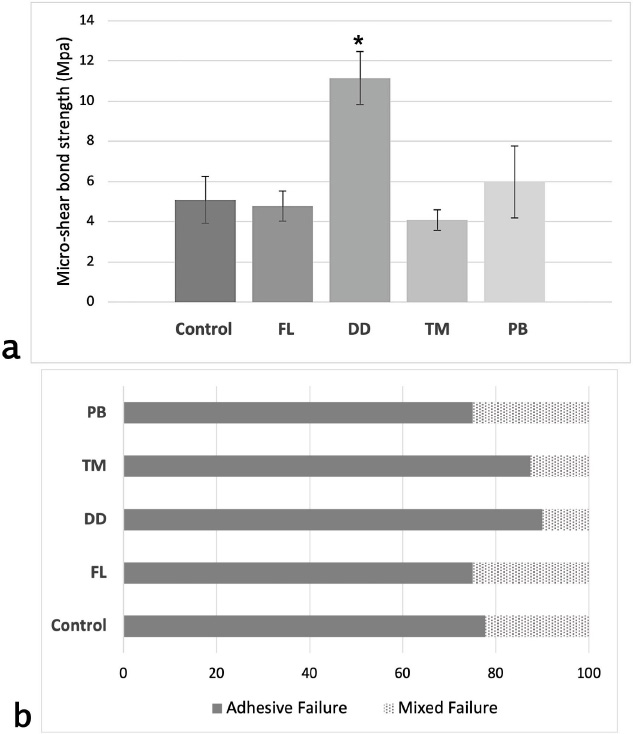
(a) Bar chart illustrating the mean micro-shear bond strength values along with standard deviations for each test group. The x-axis represents the experimental groups, while the y-axis shows the bond strength in megapascals (MPa). An asterisk (*) denotes a statistically significant difference compared to all other groups (p < 0.001). (b) Percentage distribution of failure modes across all experimental groups. The y-axis lists the test groups, and the x-axis indicates the percentage (%) of specimens exhibiting each failure mode.
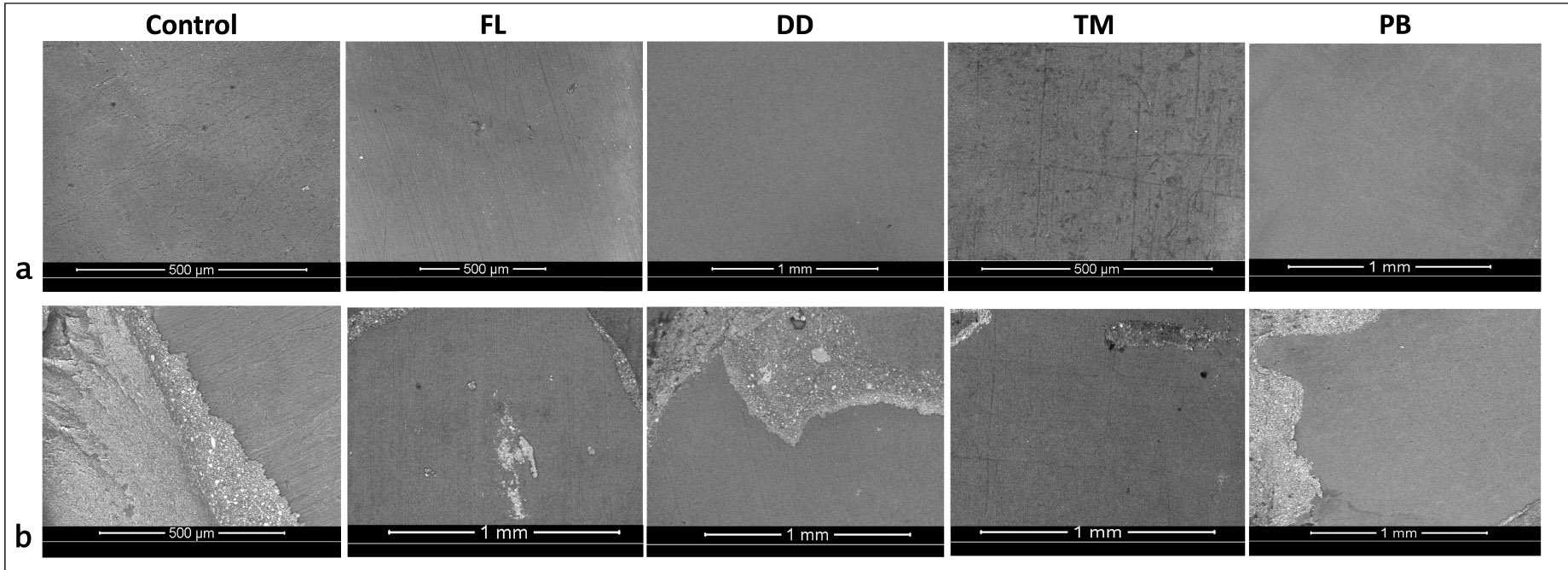
Representative Scanning Electron Microscope (SEM) images showing failure modes across experimental groups. Adhesive failure is illustrated in group (a) images, whereas (b) represent mixed type of failure.
The failure mode analysis results indicate that adhesive failure between dentin and cement was the predominant failure type across all groups (Fig. 1b). The second most common failure mode observed was mixed failure. No cohesive failures were observed within either the cement or dentin in any group. Representative SEM images of adhesive and mixed failures from each group are presented in Fig. (2). Adhesive failures, as seen in group (a) images, were characterized by smooth dentin surfaces with no resin residue, indicating superficial and insufficient bonding. In contrast, mixed failures (group b images) revealed localized areas with resin cement remnants and partially xposed dentin, suggesting partial resin infiltration and interfacial interaction.
The EDX analysis showed a notable shift in the elemental composition of dentin after applying different desensitizers. In the control group, the spectra exhibited strong peaks for Calcium (Ca) and Phosphorus (P), with Ca making up 44.97% by weight and P 19.92%. Carbon (C) percentage was 15.41%, representing the organic matrix in dentin.
In the FL group, both calcium and phosphorus levels increased, with Ca reaching 54.30% and P 22.45% by weight, while carbon levels dropped to 7.11%. In comparison, the DD group exhibited Ca and P levels of 48.88% and 23.92% by weight, respectively, and an organic profile that closely matched that of the control group.
The TM group showed a Ca content of 60.56% by weight and P at 25.79%, with a relatively lower C content at 5.15%. In the PB group, Ca levels were the highest among all groups, demonstrating a significant increase in intensity in the corresponding spectra, reaching 68.06% by weight, with a p-value of 25.81%. The C-level was the lowest compared to other groups (2.41%). Representative EDX spectra for each group are presented in Fig. (3), while the weight and atomic percentage of key elements are summarized in Table 2. To facilitate comparison of elemental composition across groups, the weight percentage data are also presented as a bar graph in Fig. (4).
4. DISCUSSION
This study compared the influence of bioactive-based desensitizers on dentin surface composition and bonding performance, using EDX analysis to detect elemental changes and to elucidate the effects on the bond strength of self-adhesive resin cement. The findings suggest that the desensitizers did not significantly affect the bond strength of self-adhesive resin cements to dentin, except in DD, where a significant increase in the bond strength was observed. EDX analysis revealed that all desensitizers increased mineralization on the dentin surface, as evidenced by increases in Ca and P, along with a reduction in C. Therefore, the null hypothesis was rejected.
In the present study, a self-adhesive resin cement was utilized, which primes the dentin surface without requiring separate etching or bonding steps. To ensure consistency, only one commonly used resin cement was selected. Self-adhesive resin cements are popular due to their straightforward application and reduced postoperative sensitivity, making them a preferred choice for a range of indirect restorations [16]. The bond strength of self-adhesive resin cements primarily depends on the interaction between acidic functional monomers, such as 10-MDP, and the calcium in the HA of dental tissues [12]. However, previous studies have indicated that the long-term performance of self-adhesive resin cements is generally low and recommended applying self-etching adhesives after preparation to seal dentinal tubules, reduce sensitivity, and improve bonding by forming a stable hybrid layer [17, 18].
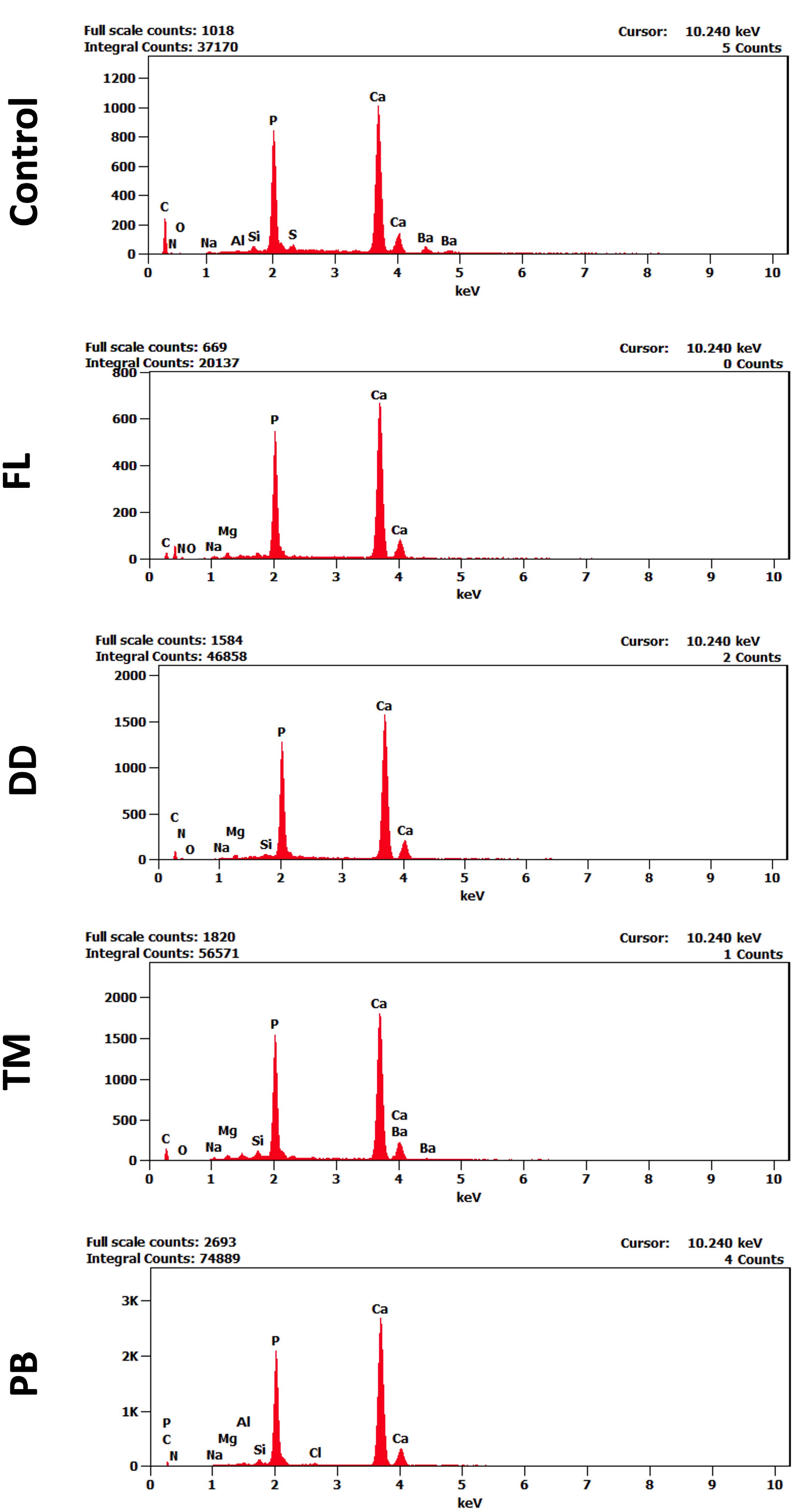
Representative EDX spectra of dentin surfaces in different study groups, showing labeled elemental peaks. Variations in peak intensities indicate changes in surface elemental composition among the treatment groups. The x-axis represents the energy levels keV, while the y-axis indicates the intensity of detected X-ray signals.
| - | - | C | N | O | Na | Mg | P | Ca |
|---|---|---|---|---|---|---|---|---|
| Control | Weight % Error (+/-1 Sigma) |
15.41 ±0.34 |
6.26 ±1.22 |
1.13 ±0.36 |
0.42 ±0.08 |
0.59 ±0.08 |
19.92 ±0.35 |
44.97 ±0.60 |
| Atom % Error (+/-1 Sigma) |
33.56 ±0.74 |
11.68 ±2.29 |
1.85 ±0.58 |
0.47 ±0.09 |
0.64 ±0.09 |
16.82 ±0.30 |
29.34 ±0.39 |
|
| FL | Weight % Error (+/-1 Sigma) |
7.12 ±0.38 |
10.15 ±1.97 |
3.04 ±0.66 |
0.82 ±0.15 |
0.84 ±0.12 |
22.45 ±0.46 |
54.30 ±0.94 |
| Atom % Error (+/-1 Sigma) |
16.03 ±0.84 |
19.60 ±3.79 |
5.14 ±1.11 |
1.21 ±0.21 |
0.93 ±0.13 |
19.60 ±0.40 |
36.64 ±0.63 |
|
| DD | Weight % Error (+/-1 Sigma) |
11.18 ±0.983 |
7.48 ±1.08 |
1.25 ±0.38 |
0.36 ±0.08 |
0.78 ±0.07 |
23.92 ±0.31 |
48.88 ±0.62 |
| Atom % Error (+/-1 Sigma) |
31.084 ±2.73 |
15.76 ±2.27 |
2.30 ±0.71 |
0.46 ±0.10 |
0.95 ±0.09 |
22.78 ±0.29 |
38.39 ±0.46 |
|
| TM | Weight % Error (+/-1 Sigma) |
5.15 ±0.20 |
3.89 ±0.83 |
0.73 ±0.27 |
0.66 ±0.09 |
0.59 ±0.13 |
25.79 ±0.31 |
60.56 ±0.59 |
| Atom % Error (+/-1 Sigma) |
19.76 ±0.53 |
7.53 ±1.61 |
1.45 ±0.54 |
0.91 ±0.12 |
0.77 ±0.17 |
26.46 ±0.32 |
48.02 ±0.47 |
|
| PB | Weight % Error (+/-1 Sigma) |
2.41 ±0.13 |
1.82 ±0.58 |
0.40 ±0.09 |
0.15 ±0.06 |
0.15 ±0.05 |
25.81 ±0.28 |
68.06 ±0.55 |
| Atom % Error (+/-1 Sigma) |
6.84 ±0.37 |
4.43 ±1.41 |
0.59 ±0.13 |
0.21 ±0.08 |
0.18 ±0.07 |
28.42 ±0.30 |
57.92 ±0.45 |
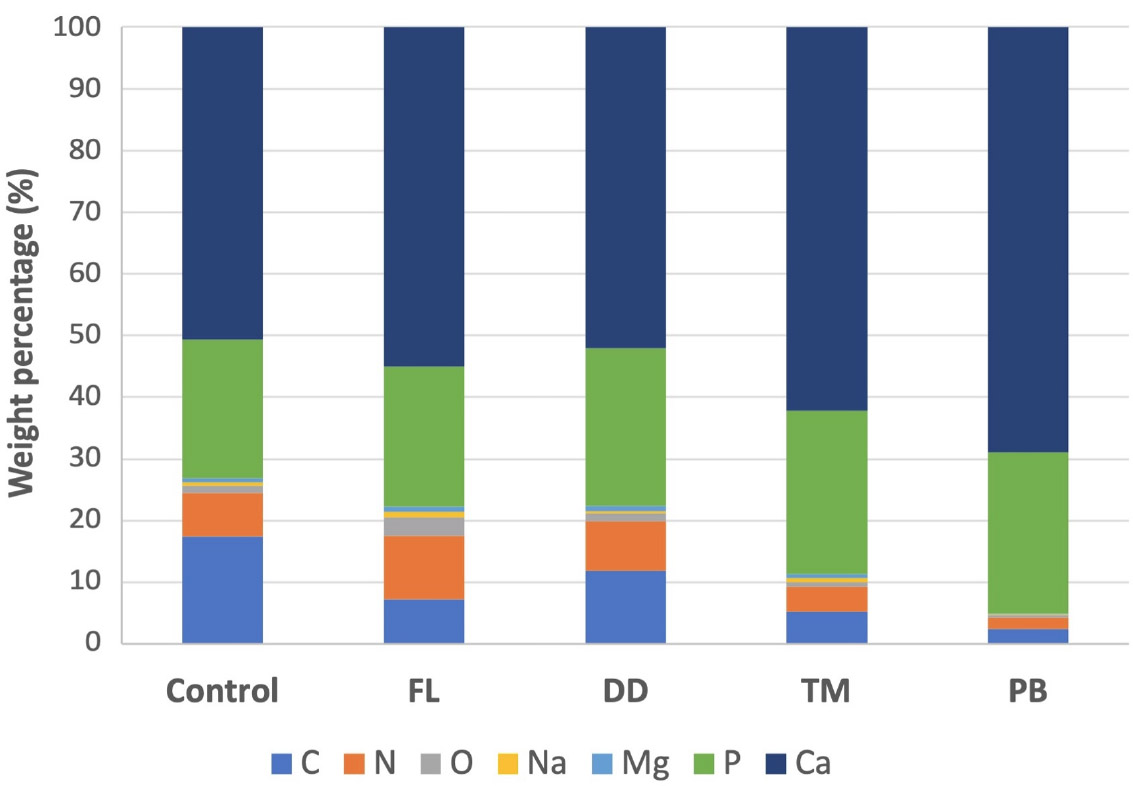
Bar graph showing the relative weight percentages of selected elements detected on dentin surfaces in each experimental group, based on EDX analysis. The x-axis represents the experimental groups (Control, FL, DD, TM, PB), and the y-axis indicates the mean weight percentage (%) of each element.
In this study, the predominance of adhesive-type failure across all test groups potentially indicates the limitations of self-adhesive resin cements in bonding to dentin. Additionally, the modification of dentin surface by different desensitizers might interfere with resin infiltration, resulting in surface-level adhesion rather than a stable hybrid layer. Self-adhesive resin cements generally lack aggressive demineralization capability and therefore may not fully compensate for the dense mineral layers formed by certain desensitizers [12, 17].
When comparing the bond strength results, specimens treated with DD showed the highest bond strength values. This suggests that DD application may have promoted favorable modification to the dentin surface, allowing for improved interaction with the resin cement. EDX analysis revealed elevated carbon content in this group, along with quantities of oxygen and nitrogen, suggesting preservation of the organic composition of the dentin. DD is a HEMA-free formulation containing 5% glutaraldehyde, fluoride, and water [19]. Glutaraldehyde cross-links with dentinal fluid protein and promotes their coagulation, effectively plugging the tubules [20]. Previous SEM analyses have demonstrated that glutaraldehyde-based desensitizers result in semi-closed dentinal tubules without forming a thick surface coating, thereby preserving surface receptivity to resin penetration and contributing to improved bond strength [21]. In addition, the interaction between glutaraldehyde and phosphate in resin cement may have contributed to improved and durable bonding [22]. These findings support previous studies, which have shown that glutaraldehyde-based desensitizers enhance bond strength by forming a stable bond interface [19, 22-24].
While DD functions through the coagulation of dentin, the other desensitizers act via mineral precipitation. In this study, FL and TM appeared to reduce the bond strength when applied to dentin before cementation. Fluoride is known to form a Calcium Fluoride (CaF2) layer on the surface of dentin [25]. The precipitated CaF2 can create a barrier that reduces the resin's ability to infiltrate and bond with dentin, leading to weaker bonding. Previous studies have reported mixed results on the influence of fluoride on bond strength. Some research indicates that the use of fluoride-containing solutions prior to bonding can reduce bond strength by interfering with hybrid layer formation and micromechanical retention. This effect was attributed to a thicker crystal layer formed on the dentin surface, as noted by Saraç et al., who concluded that higher fluoride concentrations resulted in reduced bond strength [26]. In contrast, other studies have shown that applying to demineralized dentin either increased or had no significant effect on bond strength [27, 28]. However, more recent research has consistently reported a reduction in bond strength with fluoride application [25]. In the present study, the absence of phosphoric acid etching prior to bonding may have compounded the issue, allowing CaF2 deposits and desensitizer residues to persist on the dentin surface.
Similarly, TM desensitizer demonstrated reduced bond strength. TM desensitizer stimulates the formation of HA through the reaction of its Tetracalcium Phosphate (TTCP) and Dicalcium Phosphate Anhydrous (DCPA) components. This process involves the dissolution of calcium and phosphate ions from the desensitizer, followed by re-precipitation as HA crystals [9]. Previous studies observed substantial deposit formation and tag structures in the dentinal tubules after TM treatment, with these tags primarily composed of Ca and P, indicating HA deposition within the tubules [29, 30]. While this bioactivity is effective in reducing sensitivity, it may pose a challenge for bonding [20, 29, 31]. Therefore, the reduced bond strength may be attributed to desensitizer deposition, which could obstruct dentinal tubule orifices and hinder intertubular diffusion, thereby interfering with the cement’s ability to interact and form a strong interlocking hybrid layer. This finding is consistent with the results of previous studies, which indicate that calcium phosphate desensitizers can reduce bonding efficacy by creating a physical barrier [31, 32]. However, earlier work has shown that calcium phosphate-based desensitizers did not affect bond strength when etch-and-rinse adhesives were used [33].
The EDX analysis indicated that the PB desensitizer induced the highest degree of mineralization on the dentin surface. However, this increased mineral content did not result in the highest bond strength, similar to the TM group. On the other hand, PB-treated specimens exhibited fewer adhesive failures than TM and a higher incidence of mixed failures, suggesting improved interfacial bonding. Unlike TM, PB employs a bioactive gel matrix containing Ca, P, and preformed HA. This inclusion of HA may enhance remineralization while maintaining a surface that supports bonding and chemical interaction at the interface [11, 34].
5. STUDY LIMITATIONS
This in vitro study has limitations that should be considered when interpreting the results. The controlled laboratory setting may not fully replicate the complex conditions of the oral environment. Additionally, only one self-adhesive resin cement was tested, and solely in self-etch mode, potentially limiting the generalizability of the findings. Further clinical studies and investigations involving a broader range of materials are needed to confirm and extend these findings.
CONCLUSION
Within the limitations of this in vitro study, desensitizing agents altered the dentin surface but did not consistently improve the bond strength of self-adhesive resin cement. These findings suggest that surface modifications induced by desensitizers may not uniformly benefit adhesive performance and should be considered in material selection and clinical protocols.
AUTHORS’ CONTRIBUTIONS
The authors confirm contribution to the paper as follows: A.T., R.R.B.: Study conception and design, Data collection, Analysis and interpretation of results, Draft manuscript. All authors reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| C | = Carbon |
| Ca | = Calcium |
| CaF2 | = Calcium fluoride |
| DCPA | = Dicalcium phosphate anhydrous |
| DD | = Dentin Desensitizer |
| EDX | = Energy-dispersive X-ray |
| FL | = APF Fluoride Gel |
| HA | = Hydroxyapatite |
| HEMA | = Hydroxyethyl methacrylate |
| μSBS | = Microshear bond strength |
| P | = Phosphorus |
| PB | = Predicta Bioactive Desensitizer |
| SEM | = Scanning electron microscopy |
| TM | = Teethmate Desensitizer |
| TTCP | = Tetracalcium phosphate |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Research Ethics Committee of the Faculty of Dentistry, King Abdulaziz University, under reference number 88-09-24.
HUMAN AND ANIMAL RIGHTS
All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article are available from the corresponding author [A.T] upon reasonable request.
ACKNOWLEDGEMENTS
Declared none.


