All published articles of this journal are available on ScienceDirect.
Effectiveness of Laser Therapy as an Adjunctive Treatment of Peri-Implantitis: A Systematic Review
Abstract
Introduction
Dental implants are widely used for the restoration of edentulous or partially edentulous areas of the mouth. Despite their high success rate, complications such as peri-implantitis can occur, jeopardizing implant stability. While mechanical debridement remains the standard treatment, recent evidence has suggested that adjunctive laser therapy, including photodynamic and low-level laser therapy, may offer improved outcomes. This systematic review aimed to evaluate the efficacy of laser therapy combined with mechanical debridement compared to mechanical debridement alone in the treatment of peri-implantitis.
Materials and Methods
A systematic review search was conducted in accordance with the 2020 PRISMA protocol. The search included articles published between 2019 and 2024 in English, retrieved from PubMed, Scopus, and ScienceDirect databases. After exporting the results into Microsoft Excel, articles were screened based on their titles, abstracts, and full texts to determine relevance. Only studies comparing laser-assisted therapy with conventional mechanical debridement in the context of peri-implantitis were included.
Results
A total of 90 articles were initially identified. After screening and applying the inclusion criteria, 5 studies were selected for the final analysis. These studies consistently demonstrated that adjunctive laser therapy resulted in greater improvements in clinical outcomes, including reductions in probing depth, plaque index, bleeding on probing, and bone loss, when compared to mechanical debridement alone.
Discussion
The findings of this review suggested that laser therapy, when used in conjunction with mechanical debridement, offers enhanced clinical benefits in managing peri-implantitis. The anti-inflammatory and bactericidal properties of photodynamic and low-level laser therapies appear to contribute to improved tissue healing and infection control. However, the included studies varied in terms of laser protocols, treatment durations, and follow-up periods, limiting the generalizability of the results. Standardized methodologies and longer-term follow-up are needed to validate these outcomes further and define best practices.
Conclusion
Combining laser therapy with mechanical debridement appears to be a more effective treatment strategy for peri-implantitis than mechanical debridement alone. This approach shows promise in reducing inflammation and promoting healing. Nonetheless, further high-quality research is necessary to confirm long-term benefits and establish clear clinical guidelines for its use.
1. INTRODUCTION
Dental implantology is currently a discipline with highly predictable outcomes. Its use to support prosthetic restorations has shown gratifying success in restoring function and aesthetics in patients with total or partial edentulism. However, this can also lead to various complications, whether at the implant level or in the peri-implant tissues [1].
Inflammation around a dental implant is one of the most common complications, initially affecting the peri-implant soft tissues in the case of mucositis in a reversible manner without loss of alveolar bone, unlike peri-implantitis, which is an irreversible inflammation involving both the mucosa and bone tissue adjacent to the dental implant due to bacterial contamination [2].
Common risk factors associated with this disease include poor plaque control, smoking, a history of periodontal disease, and diabetes [3].
However, the treatment of peri-implantitis relies on the decontamination of the implant surface, typically achieved through a combination of mechanical and chemical techniques, to minimize the presence of periodontal pathogenic bacteria and reduce clinical signs. In this context, many researchers have proposed using different laser techniques as an alternative approach to decontaminate infected implant surfaces [4].
The use of low-intensity laser therapy or photodynamic therapy, combined with photosensitizers, has the advantage of reducing the risk of local irritation and bacterial resistance due to its non-invasive properties [4].
While newer biomaterials such as Polyetheretherketone (PEEK) are being explored in implant dentistry, their specific role in the treatment of peri-implantitis remains under investigation. This review focuses on the effectiveness of Photodynamic Therapy (PDT) in the management of peri-implantitis associated with titanium-based implants, where a more substantial body of evidence exists.
Thus, the objective of this study is to evaluate the efficacy of laser in addition to mechanical debridement in the treatment of peri-implantitis through a systematic review, and in comparison with other treatments.
2. METHODS
2.1. Description of the Study
A systematic review study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to evaluate the efficacy of laser treatment in addition to mechanical debridement compared to conventional debridement alone.
The search for relevant articles was conducted in the electronic databases PubMed, Scopus, and ScienceDirect, as well as manually, covering the period between 2019 and 2024.
2.2. Search Strategies
2.2.1. Research Question
To target relevant articles addressing the issue, a research question was formulated according to the PICO (Population - Intervention - Comparison - Outcome) framework as follows:
- Population (P): patients with peri-implantitis.
- Intervention (I): therapeutic.
- Comparison (C): laser treatment versus mechanical debridement.
- Outcome (O): efficacy of laser in the treatment of peri-implantitis.
Therefore, the research question of this systematic review is as follows:
“Is the treatment of peri-implantitis by laser therapy effective compared to conventional mechanical debridement?”.
2.2.2. Keywords and Search Equation
The determination of the final search equation was developed from several combinations of keywords, both in English and French, presented in the Table below (Table 1).
After performing several advanced searches and using combinations of Boolean operators on the scientific databases PubMed and ScienceDirect, and Scopus, the following equation was formulated: (photodynamic therapy) OR (low level therapy) AND (mechanical debridement) AND (peri-implantitis).
| Mots Clé En Anglais | Mots Clé En Français |
|---|---|
| Peri implantitis | Péri-implantite |
| Mechanical debridement | Débridement mécanique |
| Photodynamic therapy | Thérapie photodynamique |
| Low level therapy | Thérapie laser à faible puissance |
| Non surgical treatment | Traitement non chirurgicale |
2.3. Selection Criteria
After searching and collecting the articles, they were filtered based on inclusion and exclusion criteria. All articles published between 2019 and 2024, written in the English language, and addressing photodynamic therapy or low-intensity therapy in patients with peri-implantitis were included. Meta-analyses, literature reviews, and articles not addressing photodynamic therapy or low-intensity laser therapy in patients with peri-implantitis were excluded from this systematic review.
2.4. Data Collection
After performing an advanced search in the selected databases, a total of 90 published bibliographic references were collected, including 12 from the PubMed database, 60 from ScienceDirect, 15 from Scopus, and 3 from a manual search.
The equation enabled us to collect 90 articles, which were then transferred to an Excel file for filtering by title, abstract, and finally full text.
The sorting of articles was carried out in 4 steps:
1. Elimination of duplicates
Ten duplicate results found on PubMed, ScienceDirect, and Scopus databases were deleted.
2. Sorting articles by title only
The articles were sorted using the following coding system: 1 indicated included articles, and 0 indicated excluded articles.
3. Sorting of articles according to their abstract
The articles were sorted according to the same previous coding system: 1 means inclusion and 0 means exclusion.
4. Sorting of included articles according to their full text
The articles were sorted based on their full-text availability using the previously established coding system.
5. Assessment of study quality and risk of bias
The quality of the studies included in this systematic review is essential to ensure the relevance of the results in this work. To ensure the reliability of the information provided by the included trials, the Joanna Briggs Institute JBI tool was employed, utilizing its specific checklists for randomized controlled trials.
All systematic reviews incorporate a process of criticism or appraisal of research evidence. This appraisal aimed to examine the methodological quality of a study to determine the extent to which it has addressed the possibility of bias in its design, conduct, and analysis, and to inform the synthesis and interpretation of the different results of the studies.
3. RESULTS
3.1. Flow Chart
Fig. (1) enables us to visualize the steps taken to select the relevant articles for this systematic review, thereby specifying the number of articles included and excluded, which facilitates a clear understanding of the methodology and results.
3.2. Description of the Articles Included
This section presents a synthesis of the five results from articles included in our systematic review, which compare the effectiveness of photodynamic therapy, low-power laser therapy, and mechanical debridement (Table 2).
The data from these articles are summarized in the table below, including the following information:
- The author, year, and title of the study.
- Type of study.
- The population studied.
- The objective of the study.
- A description of the study methodology.
- The results of the study.
3.3. Assessment of the Quality of the Articles selected and rIsk of Bias
The table below categorizes the quality of the five articles finally selected according to the JBI evaluation checklist (Table 3).
- Q: Question.
- Y: Yes, answer yes to the questionnaire.
- N: No, answer No to the questionnaire.
- U: Unclear, Unclear response to the questionnaire.
After assessing the scientific quality of the various articles selected through JBI, no article was excluded due to its good quality.
4. DISCUSSION
Peri-implantitis is a major inflammatory complication that affects the tissues surrounding a dental implant. Its treatment is primarily based on mechanical debridement using specialized instruments to remove plaque and tartar from the affected area.
However, mechanical debridement does not entirely eliminate the infection, especially when the probing depth exceeds 5 mm around the dental implant. For this, new complementary laser techniques have been introduced to more specifically eliminate the infection, thus optimizing the therapeutic results.
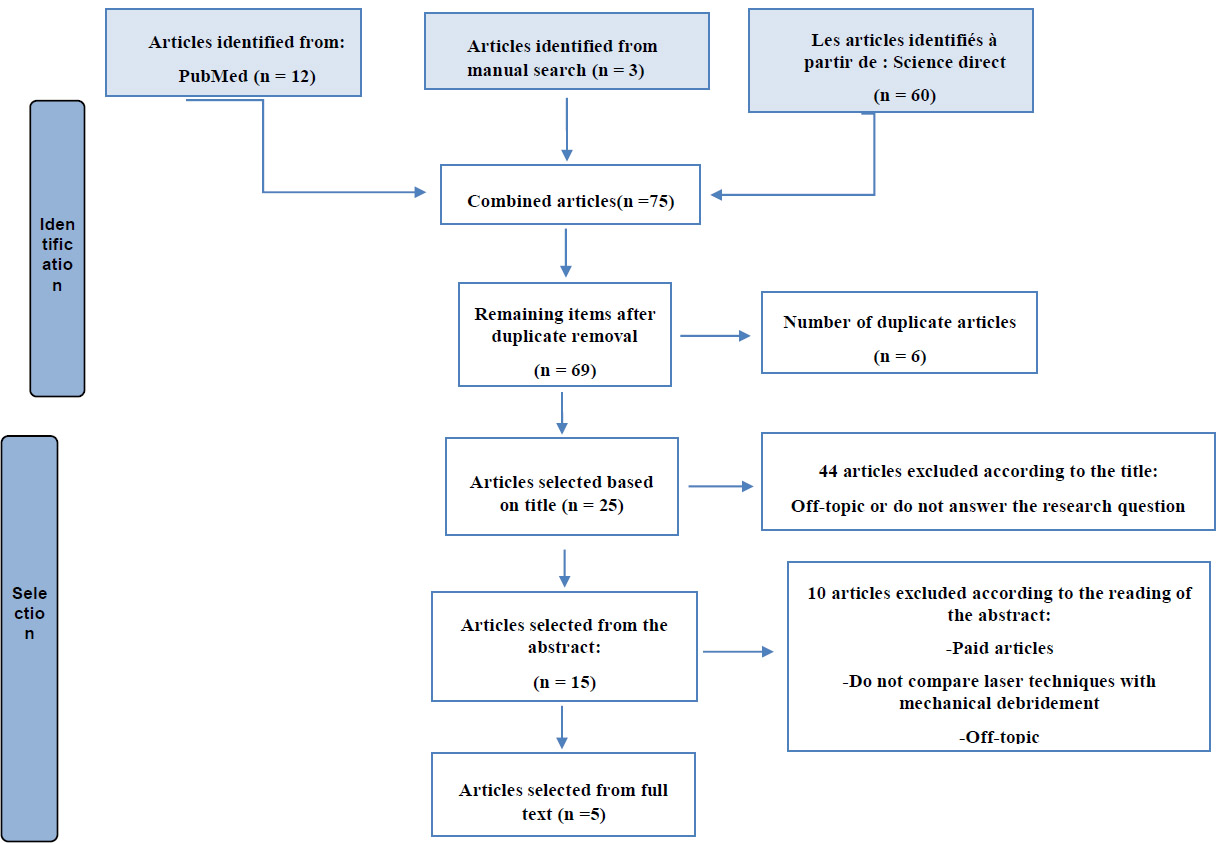
Flow diagram of the results obtained.
| Authors (year) and Title/ Refs. |
Type of Study |
Population Studied | Objective of the Study | Methodology | Results |
|---|---|---|---|---|---|
|
Alqahtani et al. (2020) Efficacy of Nonsurgical Mechanical Debridement (NSMD) With and Without Adjunct Low-Level Laser Therapy in the Treatment of Peri-Implantitis: A Randomized Controlled Trial [12]. |
A randomized controlled trial | A total of 67 patients with peri-implantitis divided into 2 groups: -33 who underwent mechanical debridement with complementary LLLT -34 who underwent NSMD alone. |
To evaluate the efficacy of LLLT as an adjunct treatment to NSMD in the treatment of peri-implantitis | The probing depth (PD), the bleeding index (BI) and even the level of bone resorption were examined in all patients at the beginning, after 3 months and after 6 months of follow-up. In the first step, a mechanical debridement was performed around the implants, followed by an LLLT by a 940 nm diode laser, a delivery of 3.41 J/cm2 and a power of 0.3 W applied perpendicular to the pocket for a duration of 20s in continuous mode and at a distance of 15 mm. |
Patients treated with mechanical debridement alone have higher clinical indices compared to patients who underwent mechanical debridement supplemented by LLLT after a follow-up of 3 to 6 months. Unlike bone resorption which does not show any statistical difference in all patients even after a follow-up of 3 to 6 months. |
|
Wang et al. (2019) Adjunctive photodynamic therapy improves the outcomes of peri-implantitis: a randomized controlled trial [5]. |
A randomized controlled trial | A total of 132 patients with peri-implantitis received mechanical debridement and photodynamic therapy (PDT) | To explore the efficacy and safety of PDT for peri-implantitis in Chinese Han patients | Patients in the control group were treated with oral cavity cleaning for 2 weeks followed by sandblasting with glycine powder and rinsing with 0.9% saline solution, while the test group received cleaning and PDT by injecting a 0.5 ml syringe of toluidine blue and 10 mg/ml of thiazine derivative at pH 3.5 into the bottom of the pocket, then each tooth was irradiated with a 635 nm diode laser and a power of 750 mW for 10s without performing any disinfection after treatment. | Patients treated with photodynamic therapy showed reduced values of clinical signs (PD/BI) at a 3-6 month follow-up compared to the control group who only underwent mechanical debridement. The PDT group showed a significant reduction in attachment loss compared to the initial value, unlike the test group which showed stable values throughout the follow-up period. |
|
Alsayed et al. (2023) Efficacy of indocyanine green and methylene blue mediated-photodynamic therapy on peri-implant outcomes among diabetics with peri-implant mucositis [6]. |
A randomized controlled trial | 60 diabetic patients with peri-implantitis were divided into 3 groups: -20 subjects received mechanical debridement -20 received Indocyanine Green (ICG) mediated photodynamic therapy in addition to mechanical debridement - 20 received Methylene Blue (MB) PDT in addition to debridement |
To evaluate the efficacy of indocyanine green-mediated photodynamic therapy versus methylene blue as an adjunct to conventional mechanical debridement in diabetic patients with peri-implant mucositis. | - Participants in the first group underwent NSMD using stainless steel curettes followed by oral hygiene motivation. - The second group underwent PDT by injecting 1 mg/ml of ICG into the pocket followed by exposure to a diode laser with a wavelength of 810 nm, a power of 200 mW and an energy of 4 J at the peri-implant sulcus. - The third group underwent PDT using 0.01% MB as a photosensitizing agent at the bottom of the pocket followed by irradiation with a 670 nm laser, a power of 140 mW and an energy of 21 J/cm2. |
- Clinical (PI/BOP/PD) and radiographic (CBL) parameters, as well as levels of pathogenic bacteria such as T. forsythia and P. gingivalis after 3 months showed significant improvement after adjuvant PDT treatment compared to mechanical debridement alone. - At the beginning of the follow-up, no difference was observed in immunological parameters (IL-6, IL-1β and TNF-α), however after 3 months a notable reduction in these values was observed. |
|
Al-Askar et al. (2022) Comparison of photobiomodulation and photodynamic therapy as adjuncts to mechanical debridement for the treatment of peri-implantitis [3]. |
A randomized controlled trial | 49 patients diagnosed with peri-implantitis divided into 3 groups: -16 received treatment by mechanical debridement alone - 16 were treated with PDT in addition to NSMD - 17 received a single session of photobiomodulation in addition to NSMD. |
Comparing the efficacy of photobiomodulation (PBMT) and PDT as an adjunct to mechanical debridement to treat peri-implantitis. | Oral cavity debridement was performed by plastic curettes followed by motivation for oral hygiene for the control group as well as the 2 test groups. Then, the first PDT test group performed a 0.05% methylene blue rinse for 5 minutes, and radiated by a 660 nm diode laser, a power of 180 mW/cm2 and an energy of 60 J/cm2. The second test group performed PBMT by a 940 nm diode laser, a power of 0.3 W and an energy of 3.41 J/cm2 in continuous mode, perpendicular to the pocket for 20 s. |
Treatment with PDT or PBMT in addition to mechanical debridement gives positive results compared to conventional treatment, this is manifested by a reduction in the plaque index, the bleeding index as well as the probing depth in patients with peri-implantitis after a 3-month follow-up. |
|
Labban et al. (2021) Clinical, bacterial, and inflammatory outcomes of indocyanine green-mediated photodynamic therapy for treating periimplantitis among diabetic patients: A randomized controlled clinical trial [7]. |
A randomized controlled trial | 48 diabetic patients were divided into two groups: - A test group received PDT treatment - A control group underwent mechanical debridemen. |
To evaluate the efficacy of indocyanine green-mediated PDT as an adjunct to NSMD compared to debridement alone in diabetic patients with peri-implantitis | - Patients in the test group underwent PDT by applying 1 mg/ml of the photosensitizer solution (ICG) to the bottom of the pocket followed by its activation by a diode laser of 810 nm, a power of 200 mW and an energy of 4 J in continuous mode for 30 s on the papilla, then 10 s on the peri-implant pocket. - Patients in the control group received debridement by an ultrasound device. |
- Significant improvement of clinical (PD/BOP and suppuration) and radiographic (CBL) signs in the indocyanine green-based PDT group compared to NSMD alone. -A remarkable decrease in bacterial loads of T. denticola and P. gingivalis in the PDT group. -A reduction in pro-inflammatory cytokines during the first months of follow-up in both groups, but their values gradually increased after 6 months. |
| Authors/Refs. | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Q13 | Score | % of Yes | Risk of Bias | Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alqahtani et al [12]. | Y | U | Y | Y | U | Y | Y | Y | Y | U | Y | Y | Y | 10/13 | 76% | Low Risk | High |
| Wang et al. [5] | Y | U | Y | N | N | Y | Y | Y | Y | Y | Y | Y | Y | 10/13 | 76% | Low Risk | High |
| Alsayed et al. [6] | Y | Y | Y | U | Y | U | U | Y | Y | Y | Y | Y | U | 9/13 | 69% | Average Risk | Average |
| Al-Askar et al. [3] | Y | Y | Y | Y | U | Y | Y | Y | Y | Y | Y | Y | Y | 12/13 | 92% | Low Risk | High |
| Labban et al. [7] | Y | Y | Y | U | Y | Y | U | Y | Y | Y | Y | Y | Y | 11/13 | 84% | Low risk | High |
This study aimed to evaluate the effectiveness of laser therapy in addition to mechanical debridement compared to conventional debridement alone, as well as in comparison to other treatment modalities, through a systematic review.
To answer the research question, this study included a total of five relevant results. These results were compared with other studies addressing the same topic to provide a comprehensive assessment of the effectiveness of different laser techniques (photodynamic therapy and low-power laser therapy) in addition to mechanical debridement, and to compare them with conventional debridement.
Indeed, studies have shown that the combination of photodynamic therapy and mechanical debridement represents an optimal therapeutic alternative for treating peri-implantitis compared to mechanical debridement alone. These effects were closely dependent on several factors, including not only the adjustment of different laser parameters but also the type of photosensitizer used. The interaction between these colorants and the laser was crucial to ensure the effectiveness of the mechanism of action of this technique [5].
In the study conducted by Wang et al. 2019, toluidine blue was chosen as a photosensitizing agent, placed at the peri-implant pocket for 3 minutes after mechanical debridement of the oral cavity, followed by its irradiation by a diode laser with a wavelength of 635 nm, a power of 0.75 W for 10 seconds at each site surrounding the dental implant [5-7]. However, Bombeccari et al. 2013 and Karimi et al. 2016 used the same photosensitizing agent (toluidine blue) at a concentration of 0.1 mg/ml but with an application duration that varied from 1 to 3 minutes [8, 9]. However, the laser used to activate this photosensitizing agent remained the same (diode laser), but with different parameters, The wavelength which varied from 630 nm to 810 nm, the power of 1 W, the power density of 2,000 mW/cm and the total exposure time which could range from 1 to 2 minutes [8, 9].
On the other hand, methylene blue has been chosen as a photosensitizing agent in other studies such as the Romeo et al. [10] study in 2016 used a concentration of 10 mg/ml for 1 minute at the peri-implant pocket, followed by its activation by a diode laser with a wavelength of 670 nm and a power density of 75 mW/cm 2, while Almohareb et al. 2020 [11], only mentioned the photosensitizer insertion period of 1 minute and its exposure by a 660 nm diode laser for 10 seconds per site, without specifying its concentration.
In addition, the study conducted by Labban et al. in 2021 aimed to evaluate the efficacy of PDT combined with conventional debridement using indocyanine green as a photosensitizing agent in diabetic patients [7]. A concentration of 1 mg/mL of Indocyanine Green (ICG) was applied for 1 minute to the peri-implant pocket, followed by diode laser exposure at a wavelength of 810 nm, a power of 0.2 W, and a total energy of 4 J. The results of this study revealed a significant improvement in clinical and radiographic parameters in diabetic patients treated with ICG-mediated PDT compared to conventional treatment. In addition, a decrease in the levels of Porphomonas gingivalis and Treponema denticola was observed after a follow-up of 3 to 6 months, unlike patients treated only with mechanical debridement, who failed to maintain the reduction of these bacterial species up to 6 months of follow-up.
Furthermore, the study by Alsayed et al. 2023 indicated that the results of treatment with photodynamic therapy based on indocyanine green are comparable to those based on methylene blue, thereby reducing the level of bacteria present in the environment as well as clinical and radiographic signs in diabetic patients suffering from peri-implantitis [6].
It should be noted that other factors may influence the results of PDT treatment, in addition to mechanical debridement, including the laser fiber diameter and fluence, which have not been extensively studied. Determining the amount of energy delivered during the PDT process is more complex [12]. However, Romeo et al. 2016 and Bombeccari et al. 2013 mentioned that a diameter between 0.03 and 0.06 cm and a fluence of 25.54 J/cm2 are the most appropriate parameters for this type of treatment [8-10].
The study by Wang et al. 2019 also proved that the combination of photodynamic therapy with mechanical debridement allowed a reduction in various clinical parameters, including probing depth, which showed a significant decrease after 3 months of follow-up and even more after 6 months in patients treated with photodynamic therapy compared to patients who only performed mechanical debridement by cleaning and sandblasting with glycine powder [5]. In this context, Karimi et al. 2016 and Romeo et al. 2016 obtained the same results regarding probing depth, specifying that the latter showed minimal results at the beginning of the study for both the photodynamic therapy group and the group that performed mechanical debridement by scaling or air polishing with glycine powder [9, 10]. These two studies showed that after 3 months, the results began to become increasingly positive, and even more so after 6 months of follow-up, as indicated in the study by Wang et al. Regarding bleeding on probing, the study by Wang et al. 2019 showed that most patients at the beginning of follow-up have grade 4 bleeding on probing. After treatment with mechanical debridement followed by photodynamic therapy, the results became positive, showing bleeding on probing with a grade 1, unlike patients treated with mechanical debridement alone, who reported a bleeding index of grade 3, even after a 6-month follow-up [5]. In this connection, Karimi et al. 2016 and Romeo et al. 2016 shared the same vision and specified that the bleeding index on probing and suppuration became zero after the use of photodynamic therapy, as opposed to the site treated only by mechanical debridement, where bleeding was still present after peri-implant probing [9, 10].
The study by Alqahtani et al. 2020 showed that the application of Low-Level Laser Therapy (LLLT) in addition to mechanical debridement contributed to a decrease in probing depth as well as the level of crestal bone resorption, compared to mechanical debridement alone. These results were obtained by adjusting the diode laser parameters to a wavelength of 940 nm, an energy flux of 3.41 J/cm2, and a power of 0.3 W for 20 seconds at the peri-implant pocket [12]. Both techniques, respectively, low-power laser therapy and photodynamic therapy, used in combination with mechanical debridement, seemed to produce encouraging results for the treatment of peri-implantitis [13-16]. However, the study by Tonin et al. [1], demonstrated that photodynamic therapy is more effective than low-level laser therapy, with a 44.41% reduction in Staphylococcus aureus and a 46.14% reduction in periodontal biofilm [17-20].
Recent studies have suggested that combining laser therapy with chemical debridement, Photodynamic Therapy (PDT), and even surgical procedures can yield superior results compared to using each modality individually. The synergistic effects of these combinations could address both bacterial infection and tissue regeneration more effectively, providing more comprehensive management of peri-implantitis.
4.1. Combination of Laser Therapy and Photodynamic Therapy (PDT)
Studies have demonstrated that laser therapy, when combined with PDT, may enhance bacterial eradication, promote tissue regeneration, and reduce inflammation more effectively than when each treatment is applied independently. Laser therapy helps decontaminate the implant surface and improve tissue response, while PDT adds an antimicrobial effect by utilizing light-activated photosensitizers to target bacterial biofilms. This combination can significantly reduce the recurrence rates of peri-implantitis [21, 22].
4.2. Laser Therapy and Chemical Debridement
Similarly, laser therapy combined with chemical agents, such as chlorhexidine or hydrogen peroxide, has been proposed as an effective strategy. The laser decontaminates the surface and reduces the bacterial load, while chemical agents further help to disinfect the peri-implant tissues. This combination can potentially provide both immediate bacterial control and long-term prevention of further bacterial colonization, particularly when used in conjunction with mechanical debridement [23].
4.3. Laser Therapy and Surgical Intervention
The combination of laser therapy and surgical interventions, such as open flap debridement or implantoplasty, can also provide synergistic benefits. Surgical interventions, while effective, are invasive and carry risks of complications. When combined with laser therapy, however, there is a potential for improved wound healing and a reduction in the extent of tissue trauma. The laser can be used to clean the implant surface, promote tissue regeneration, and aid in better soft tissue management during surgical procedures [24].
Additionally, the combination of photodynamic therapy and mechanical debridement proved to be more efficient in treating peri-implantitis than mechanical debridement alone. In contrast, Al-Askar et al. found that both photodynamic therapy and low-level laser therapy showed similar efficacy in reducing clinical and radiological parameters of peri-implantitis [3]. However, they did not establish a clear preference for one treatment method. Given its superior bactericidal effects, photodynamic therapy should be considered the preferred adjunctive treatment to mechanical debridement for effectively managing peri-implantitis [14-23].
While laser therapy has demonstrated promising results in managing peri-implantitis, its application is not without potential drawbacks. One of the primary concerns is the risk of thermal damage to the peri-implant bone, particularly when high-energy lasers, such as Nd: YAG or CO2 lasers, are used without adequate cooling. A temperature rise above 47°C for more than one minute can result in irreversible bone necrosis, compromising osseointegration and potentially leading to implant failure. Moreover, inappropriate laser settings can alter the implant surface morphology, causing melting, cracking, or smoothing of the microstructured titanium surface, thereby reducing its capacity to support bone integration and increasing the risk of bacterial colonization. Additionally, disruption of the protective titanium oxide layer may negatively affect the biocompatibility of the implant, promoting corrosion or inflammatory responses. Misapplication of laser energy may also cause injury to surrounding soft tissues, resulting in gingival recession or delayed wound healing. Furthermore, when laser decontamination is insufficient, residual biofilms may persist, undermining the clinical efficacy of the treatment. These limitations highlight the importance of precise control over laser parameters and the careful selection of suitable laser systems to achieve therapeutic benefits while minimizing iatrogenic complications [24-26].
Mechanical debridement combined with laser therapy is commonly used in the treatment of peri-implantitis to remove biofilm and bacteria from the implant surface. While these treatments can effectively reduce inflammation and promote tissue healing, concerns have been raised regarding the potential impact on the stability of the dental implant. Here's a discussion on how mechanical debridement and laser therapy may influence implant stability.
4.4. Mechanical Debridement and Implant Stability
Mechanical debridement is a process that involves the physical removal of bacterial biofilm and calculus from the implant surface using instruments such as curettes, ultrasonic scalers, or abrasives. While this procedure is effective in cleaning, improper technique or overuse of abrasive instruments can potentially cause damage to the implant surface. Excessive mechanical stress can lead to microfractures or surface roughness, which may affect osseointegration—the process by which the implant fuses with the surrounding bone.
However, when performed correctly and gently, mechanical debridement is considered safe and has minimal effect on the long-term stability of the implant. The goal is to clean the implant without damaging the bone-implant interface, which is crucial for maintaining stability [27, 28].
4.5. Laser Therapy and Implant Stability
Laser therapy, particularly in the form of Er: YAG (Erbium-doped Yttrium Aluminum Garnet) lasers, has become a popular adjunctive treatment for peri-implantitis due to its ability to decontaminate the implant surface while minimizing damage to surrounding tissues. Lasers, when appropriately used, can effectively remove bacterial biofilm and promote tissue regeneration, thereby reducing the risk of implant failure due to infection.
However, concerns about laser use often stem from the potential for heat generation. Excessive heat buildup can cause thermal damage to the surrounding bone and soft tissues, potentially compromising implant stability. Therefore, it is essential to use the appropriate laser settings (power, wavelength, and pulse duration) to avoid excessive heat accumulation. Studies have shown that when laser therapy is applied correctly, there is no significant adverse effect on the stability of the implant [29, 30].
4.6. Combined Mechanical Debridement and Laser Therapy
Combining mechanical debridement and laser therapy may offer synergistic benefits, enhancing bacterial eradication while minimizing tissue damage. The mechanical instruments can physically clean the implant surface, while laser therapy further decontaminates and promotes healing. This combination, when used cautiously, is unlikely to negatively affect implant stability, provided that the techniques are applied correctly and with appropriate care.
Both mechanical debridement and laser therapy, when performed with proper technique, do not appear to affect the stability of dental implants significantly. However, it is crucial to avoid excessive force during mechanical debridement and to manage laser settings to prevent thermal damage carefully. Proper technique is crucial in maintaining the stability of the implant while effectively treating peri-implantitis [30-33].
CONCLUSION
In conclusion, Photodynamic Therapy (PDT) has emerged as a highly promising and minimally invasive treatment modality for peri-implantitis. Its effectiveness lies in its ability to target and eradicate bacterial biofilms, which are often a significant cause of peri-implantitis and contribute to treatment failure. The light-activated photosensitizers used in PDT provide an antimicrobial effect without the need for extensive surgical intervention, making it an ideal option for patients seeking a less invasive treatment alternative. Additionally, PDT has been shown to significantly reduce inflammation, promote tissue regeneration, and improve overall healing around the implant, all while being well-tolerated by the patient.
When combined with mechanical debridement, PDT becomes even more powerful. Mechanical debridement, which involves physically removing biofilm and contaminated tissue from the implant surface, is often an essential first step in treating peri-implantitis. However, it may not be sufficient to fully address the microbial component of the disease, particularly in cases of advanced peri-implantitis. The addition of PDT as an adjunctive treatment enhances the therapeutic effect by providing an additional layer of bacterial eradication and accelerating tissue healing. This combined approach, when performed with care and precision, yields exceptional results in the management of peri-implantitis, thereby minimizing the need for more invasive surgical procedures, such as flap surgeries or implantoplasty.
Furthermore, the combination of PDT and mechanical debridement has the potential to reduce the risk of peri-implantitis recurrence. By thoroughly decontaminating the implant surface and targeting persistent bacterial colonies, these treatments work synergistically to create an environment conducive to optimal healing and long-term implant success. The minimally invasive nature of this combined treatment is a significant advantage for patients, as it reduces both the discomfort and recovery time typically associated with more invasive procedures.
Several studies have compared the clinical outcomes of mechanical debridement alone versus its combination with laser therapy in the treatment of peri-implantitis. Evidence suggests that while mechanical debridement plays a fundamental role in disrupting biofilm and removing granulation tissue, it may not fully eradicate pathogens, particularly in the microstructured surfaces of dental implants. The adjunctive use of laser therapy, particularly Er:YAG and diode lasers, has demonstrated improved decontamination of implant surfaces, reduced probing depths, and enhanced soft tissue healing. These combined effects often result in superior clinical improvements compared to mechanical debridement alone. Nevertheless, the degree of benefit can vary depending on the laser type, power settings, and the operator's expertise. Thus, while the combined therapy appears more effective in many cases, further high-quality randomized controlled trials are warranted to conclusively establish the magnitude of this benefit and standardize treatment protocols.
In summary, photodynamic therapy, particularly when used as an adjunctive treatment to mechanical debridement, represents one of the most effective, minimally invasive, and patient-friendly approaches for treating peri-implantitis. This combination not only yields successful outcomes but also holds the potential to significantly enhance clinical management and improve long-term success rates in peri-implantitis treatment.
STUDY LIMITATIONS
While this systematic review provides valuable insights into the effectiveness of laser treatments for peri-implantitis, several limitations must be acknowledged. Firstly, the included studies varied widely in their methodologies. This included differences in laser parameters such as wavelength, power, and exposure time, as well as the use of different photosensitizing agents. Such variability makes it challenging to draw definitive conclusions about the optimal laser settings or which photosensitizer is most effective in improving treatment outcomes.
Another key limitation is that many studies did not report important details about laser parameters, like the fiber diameter and energy fluence. These factors are critical because they directly influence the amount of energy delivered during the treatment. Yet, their omission makes it difficult to assess how these elements might have impacted the results. Additionally, the follow-up periods across the compared studies were inconsistent, with some studies only monitoring patients for a few months while others extended the follow-up to six months or longer. Shorter follow-up periods limit the ability to evaluate the long-term effectiveness and sustainability of laser treatments, particularly in terms of clinical improvements over time.
The outcome measures used across the studies were also not always standardized. While some studies focused on probing depth and bleeding on probing, others assessed bacterial reduction or other clinical signs. This lack of consistency in how outcomes were measured makes it harder to compare findings across different studies or to identify a clear, universally accepted set of indicators for treatment success.
Lastly, while some studies compared different types of laser treatments (e.g., low-level laser therapy vs. photodynamic therapy), others only compared laser treatments to mechanical debridement. This lack of uniform comparison methods makes it difficult to evaluate which laser treatment, if any, is superior for managing peri-implantitis.
Despite these limitations, the findings of this review underscore the potential of laser therapies as adjuncts to mechanical debridement for treating peri-implantitis. However, further studies with more standardized protocols, more extended follow-up periods, and a broader range of patient factors are needed to strengthen the evidence base and provide more conclusive recommendations.
AUTHORS’ CONTRIBUTIONS
It is hereby acknowledged that all authors have accepted responsibility for the manuscript's content and consented to its submission. They have meticulously reviewed all results and unanimously approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| CAD/CAM | = Computer-Aided Design / Computer-Aided Manufacturing (mentioned indirectly; may be relevant if PEEK prosthetics are discussed further) |
| CO2 | = Carbon Dioxide (used in CO2 lasers) |
| ICG | = Indocyanine Green |
| JBI | = Joanna Briggs Institute |
| LLLT | = Low-Level Laser Therapy |
| Nd | = YAG Neodymium-Doped Yttrium Aluminum Garnet |
| P | = Population (used in PICO framework) |
| PDT | = Photodynamic Therapy |
| PEEK | = Polyetheretherketone |
| PICO | = Population, Intervention, Comparison, Outcome |
| PRISMA | = Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| Q | = Question (used in JBI checklist coding) |
| U | = Unclear (used in JBI checklist coding) |
| Y | = Yes (used in JBI checklist coding) |
| N | = No (used in JBI checklist coding) |
AVAILABILITY OF DATA AND MATERIALS
All the data and supportive information are provided within the article.
ACKNOWLEDGEMENTS
Declared none.


