All published articles of this journal are available on ScienceDirect.
A Novel Technique for Vertical Bone Augmentation Using "N-Shaped" Titanium Mesh with Simultaneous Implant Placement in the Posterior Mandible: A Case Report
Abstract
Introduction
Guided Bone Regeneration (GBR) is a widely utilized technique for bone augmentation in dental implantology.
Case Presentation
This case report introduces a novel vertical bone augmentation method using an “n-shaped” titanium mesh fixed onto a dental implant via an improved healing abutment. The technique was applied to address vertical and horizontal bone defects in the posterior mandible, achieving simultaneous alveolar bone augmentation with favorable aesthetic and functional outcomes. Two years post-restoration, the vertical bone gain remained stable.
Conclusion
This implant-supported tent technique, utilizing titanium mesh, provides a minimally invasive, cost-effective, and reliable solution for vertical osteogenesis in patients with mandibular posterior bone defects.
1. INTRODUCTION
The restoration of implants in the posterior mandible with insufficient vertical bone height remains a significant challenge in implant dentistry [1, 2]. Adequate alveolar bone volume and quality are essential for ensuring long-term functional and aesthetic success [2]. Guided Bone Regeneration (GBR) has emerged as a reliable method for bone augmentation in conjunction with implant placement, particularly for localized alveolar defects [3, 4].
Various techniques, including interpositional osteotomies, onlay/inlay grafts, and alveolar distraction osteogenesis, have been developed to achieve stable osteogenic spaces [5]. Non-resorbable membranes, fixation screws, and titanium mesh are commonly used to secure graft materials [6]. Among these, titanium mesh has gained prominence due to its superior mechanical properties and biocompatibility, enabling its use in larger or vertical bone defects [7].
A critical factor in GBR success is the maintenance of a stable, secluded space beneath the membrane, which directly influences the quantity and quality of newly formed bone [6, 8]. However, in cases of vertical and horizontal bone defects, the lack of supporting bone walls often leads to membrane collapse under soft tissue pressure, compromising osteogenesis [9].
To enhance the support and stabilization of the membrane and to augment the osteogenic potential at GBR sites, several strategies have been proposed. These include the utilization of reinforced expanded Polytetrafluoroethylene (e-PTFE) membranes or the application of mini screws and pins [5, 10, 11]. Additionally, the adoption of self-reinforced polyglycolide membranes has been recommended. However, the potential for lateral collapse of the membrane exists, prompting the suggestion of employing various graft types to preserve the space between the implant and the surrounding defect [12, 13].
For addressing the vertical component of ridge deformities, titanium micromesh membranes have been advocated [14]. The incorporation of titanium mesh has broadened the scope of GBR applications, attributed to its superior mechanical properties and biocompatibility. This advancement enables the application of GBR technology in the reconstruction of alveolar ridges with significant bone deficiencies or vertical bone loss, yielding superior and consistent bone augmentation outcomes [15].
Presently, GBR utilizing titanium mesh is employed in a variety of clinical scenarios, encompassing diverse procedural approaches [16]. Options are available regarding bone graft materials, techniques for covering with titanium mesh, and methods for securing the titanium mesh [14]. The fixation method for the titanium mesh can be tailored based on the extent of bone augmentation required and the decision to perform simultaneous implant placement. Titanium screws are commonly used to secure the titanium mesh, which can later be removed during subsequent implant surgery [7].
In cases of single tooth loss requiring titanium mesh bone augmentation, a simultaneous implantation approach may utilize absorbable sutures for mesh fixation. This method circumvents the interference of titanium screw placement with implants. It eliminates the need for additional incisions to remove the titanium mesh and screws in subsequent surgeries, thereby minimizing patient trauma [16].
This report presents a novel implant-supported tent technique using an “n-shaped” titanium mesh fixed onto a dental implant via a modified healing abutment. The technique was applied to a patient with vertical bone deficiency in the posterior mandible, demonstrating successful bone augmentation and implant restoration.
2. CASE REPORT
A 23-year-old female presented to the Department of Stomatology, The Seventh Medical Center of PLA General Hospital, for implant restoration of the left mandibular first molar (tooth 36), which had been extracted two years prior due to periapical periodontitis.
This study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the Joint Committee on Clinical Investigation of The Seventh Medical Center of PLA General Hospital (Beijing, China). The patient received detailed information about the examinations in advance and provided written consent to participate in the study.
2.1. Clinical Examination
The patient demonstrated good oral hygiene, with a FMPS of 18% and an FMBS of 10%. The edentulous site showed buccal and vertical alveolar bone defects, with no adjacent tooth inclination or opposing tooth elongation (Fig. 1a, 1b).
2.2. Imaging Examination
Cone-Beam Computed Tomography (CBCT) revealed a 4 mm vertical bone defect and an alveolar crest width of 6.6 mm at the edentulous site. The distance from the alveolar crest to the mandibular canal was 13.2 mm (Fig. 1c, 1d). The third molar (tooth 38) was mesioangularly impacted.
2.3. Treatment Design
Given the patient's high aesthetic and functional demands, an implant-supported tent technique with titanium mesh was proposed. This approach aimed to achieve simultaneous vertical bone augmentation and implant placement, minimizing surgical trauma and treatment duration.
2.4. Brief Introduction of the Implant-supported Tent Technique with Titanium Mesh Shelter
A modified healing abutment was developed to secure a prefabricated titanium mesh on top of the dental implant, ensuring a stable and isolated space beneath the membrane to facilitate osteogenesis in both vertical and horizontal bone defects (Fig. 2a-c).
This modified healing abutment has been granted a patent under the number ZL2017 21817499.0 [17].
2.5. Instruments and Consumables
- BASICKIT dental implantation tool group (BASIC Omni-Tight, USA)
- BASIC bone compression tool group (BASIC Omni-Tight, USA)
- BASIC 4.5×13 mm dental implant system (BASIC omni-tight, USA) (Fig. 2d)
- Bone graft materials 0.5 cc (Bio-Oss, Geistlich, Switzerland) (Fig. 2e)
- Resorbable collagen membrane (Bio-Gide, Geistlich, Switzerland) (Fig. 2e)
- Titanium mesh (ACE Surgical Supply Company, USA, aperture 1 mm, thickness 0.1 mm) (Fig. 2e)
- Modified healing abutment
2.6. Preoperative Preparation
Preoperative Cone Beam Computed Tomography (CBCT) was conducted to assess the bone morphology and defect dimensions. Implant placement planning was meticulously designed using specialized implant planning software. Subsequently, a surgical guide template was fabricated using a Rapid Prototyping (RP) technique to ensure precise implant positioning [18].
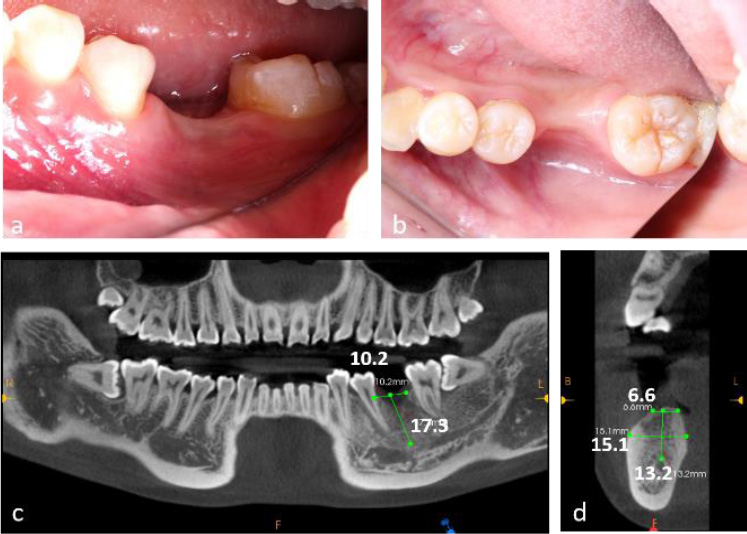
Preoperative clinical and radiographic evaluation of a 23-year-old female patient presenting with tooth loss (#36) and concomitant alveolar bone atrophy. (a) Intraoral clinical photograph demonstrating buccal view of the edentulous area, (b) Intraoral clinical photograph showing occlusal view of the defect site, (c) Preoperative cone-beam computed tomography (CBCT) scans, (d) CBCT cross-sectional analysis revealing alveolar crest width of 6.6 mm and basal bone width of 15.1 mm at the edentulous site.
Antibiotic prophylaxis was initiated with amoxicillin/clavulanic acid (Augmentin, GlaxoSmithKline, Verona, Italy; 2 g administered 1 hour prior to surgery and continued at 2 g per day for 8 days). Additionally, the patient was instructed to perform mouth rinses with 0.2% chlorhexidine (Corsodyl, GlaxoSmithKline) starting preoperatively and continuing for 2 weeks postoperatively. To manage postoperative pain and inflammation, nimesulide 100 mg (Aulin, Roche, Milano, Italy) was administered 1 hour before surgery and twice daily for 7 days.
2.7. Intraoperative Surgical Procedures
Local anesthesia (Scandonest 2%, Septodent, Saint-Maur-des-Fosses Cedex, France) was administered for hemostasis. The implant surgical guide template and a novel computer-assisted drill guide template were applied to confirm the implant site (Fig. 3a).
A mid-crestal full-thickness incision was created and extended to teeth 45 and 47 through an intrasulcular incision. Two vertical incisions were performed to prepare a trapezoidal mucoperiosteal flap. The flap was elevated on both the buccal and lingual sides to expose the alveolar crest of the molar space. The depth of the implant socket was 13 mm and confirmed by a guiding drill (the occlusal surface was flush with the crest of the adjacent buccal alveolar ridge) (Fig. 3b).
The implant socket was prepared using osteotomes. A periodontal probe was used to check the integrity of the bone wall, and granulation tissue in the implant socket was completely removed.
Bleeding points (decortication) were created using a round bur to expose the underlying marrow of the alveolar crest. The implant socket was filled with bone material (Bio-Oss, Geistlich, Switzerland). Subsequently, the implant (BASIC 4.5×13 mm) was implanted with a torque of 25 N·cm. A periodontal probe was used to examine the exposure of the implant, with 4 mm on the buccal side and 2 mm on the lingual side (Fig. 3c-i).
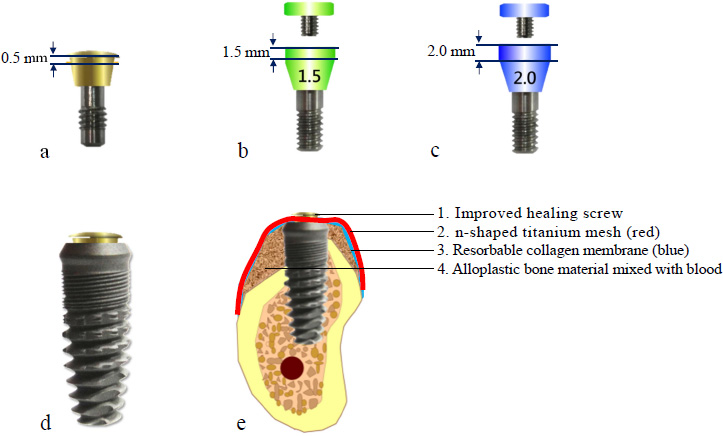
Design characteristics of modified healing abutments and titanium mesh application. (a) Modified healing abutment with 0.5 mm vertical height, (b) Modified healing abutment demonstrating 1.5 mm vertical dimension, (c) Modified healing abutment with 2.0 mm vertical profile, (d) Clinical application of 0.5 mm healing abutment in conjunction with dental implant, (e) Schematic representation of implant-supported tenting technique utilizing titanium mesh for guided bone regeneration.
2.8. Pre-shaping of Titanium Mesh
The titanium mesh was customized according to the dimensions of the osteogenesis area, measuring 12 mm in height and 22 mm in width. The mesh was trimmed to accommodate the buccal height (10 mm), occlusal surface (8 mm), and lingual side (4 mm). It was then contoured into an “n” shape and trial-fitted to ensure proper adaptation (Fig. 3j). A hole was drilled into the occlusal surface for placement of a modified healing abutment.
2.9. Placement of Titanium Mesh
Bone graft material was applied around the implant neck and the buccal alveolar bone defect. A bioresorbable membrane was then positioned to cover the osteogenesis area and perforated at the implant's using a probe. The titanium mesh was subsequently placed and secured onto the implant using a 0.5 mm modified healing abutment (Fig. 3g-i).
Tension-free closure of the soft tissue using resorbable suturing material (Vicryl 3-0) could not be achieved due to insufficient soft tissue availability. As a result, the titanium mesh remained partially exposed, and a periodontal dressing was applied to protect the surgical site (Fig. 3k).
2.10. Removal of Titanium Mesh
Approximately two months postoperatively, soft tissue proliferation and epithelization were observed beneath the exposed titanium mesh. Despite the presence of debris and muck adhering to the mesh, no clinical signs of inflammation were detected (Fig. 4a).
During the re-entry procedure, the titanium mesh was found to be encased in dense connective tissue. The mesh was removed six months after the initial surgery, revealing that the space beneath it was occupied by tissue exhibiting macroscopic characteristics of newly formed bone (Fig. 4b, 4c).
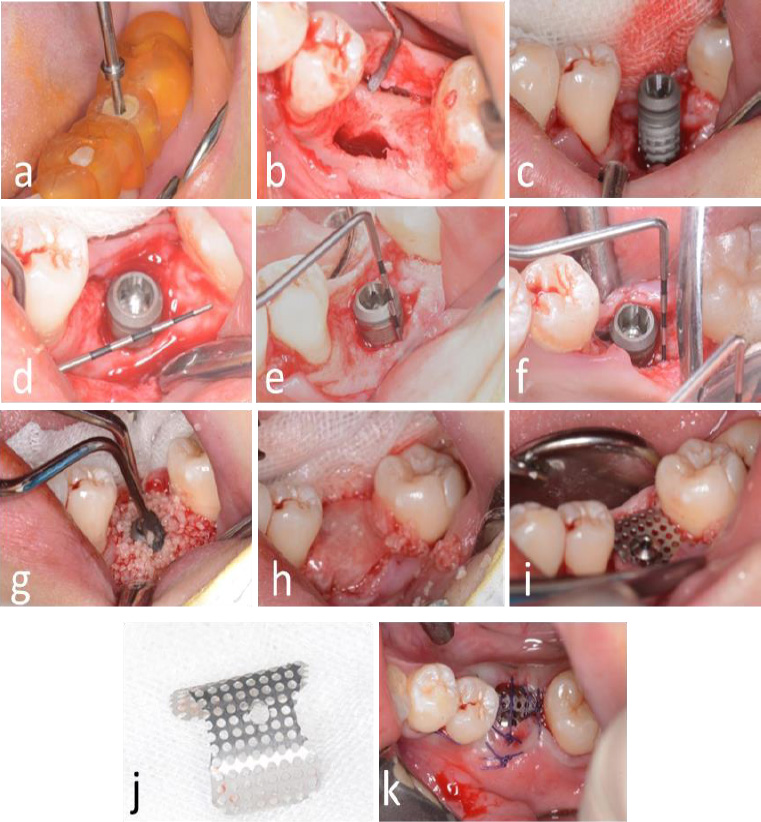
Surgical procedure for dental implant placement. (a) Positioning of the surgical guide template, (b) Reflection of a trapezoidal mucoperiosteal flap, (c) Osteotomy and implant placement, (d) Peri-implant bone defect at the cervical region, (e) Buccal vertical bone defect measuring 4 mm; (f) Lingual vertical bone defect measuring 2 mm, (g) Augmentation using alloplastic bone graft material, (h) Application of bioresorbable membrane to cover the graft site, (i) Fixation of titanium mesh using a 0.5 mm modified healing abutment, (j) Contouring of titanium mesh prior to placement, (k) Postoperative exposure of titanium mesh.
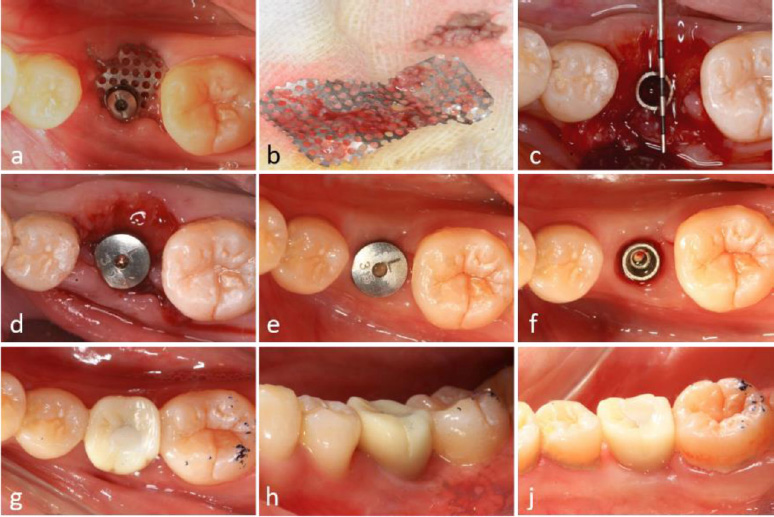
Surgical procedures and clinical outcomes of second-stage implant surgery. (a) Intraoral view at 6-month postoperative follow-up, (b) Removal of titanium mesh demonstrating residual debris accumulation, (c) Significant alveolar ridge augmentation and soft tissue formation beneath the titanium mesh, (d) Placement of healing abutment for soft tissue conditioning, (e) Peri-implant soft tissue maturation at 4 months post-healing abutment placement, (f) Establishment of well-contoured peri-implant gingival architecture, (g-i) Intraoral clinical photographs illustrating final prosthetic rehabilitation outcomes.
2.11. Alveolar Ridge Augmentation
A significant increase in both the width and height of the alveolar ridge was achieved. A transmucosal healing abutment was placed for two weeks, followed by the completion of a single-tooth implant restoration, resulting in satisfactory aesthetic and functional outcomes (Fig. 4d-4i). Postoperatively, the vertical bone height on the buccal side increased by 5.1 mm and on the lingual side by 3.0 mm (Fig. 5a-5f). Slight reductions in these measurements were observed upon completion of the implant restoration, with the buccal and lingual vertical bone heights measuring 4.4 mm and 2.0 mm, respectively (Fig. 5b, 5e).
At the 2.5-year follow-up, the vertical bone height on the buccal side was maintained at 3.3 mm, while the lingual side measured 1.9 mm (Fig. 5c, 5f).
2.12. Clinical Outcomes
Partial exposure of the titanium mesh occurred during the application of the novel implant-supported tent technique. However, this did not adversely affect the bone regeneration process. Clinically and radiographically, a significant increase in alveolar ridge width and height was confirmed. The patient demonstrated both functional and aesthetic success throughout the 2.5-year follow-up period.
4. DISCUSSION
4.1. GBR and Titanium Mesh
Secondary absorption and atrophy of alveolar bone often occur after tooth loss, leading to a reduction in the width and height of the alveolar ridge, which may be insufficient for implantation. Therefore, reconstruction of alveolar bone in the implant area is a top priority for dental implants.
Guided Bone Regeneration (GBR) is generally considered the gold standard method for reconstructing alveolar bone defects due to its simplicity, low technical sensitivity, osteogenic stability, and multidirectional osteogenesis ability [4].
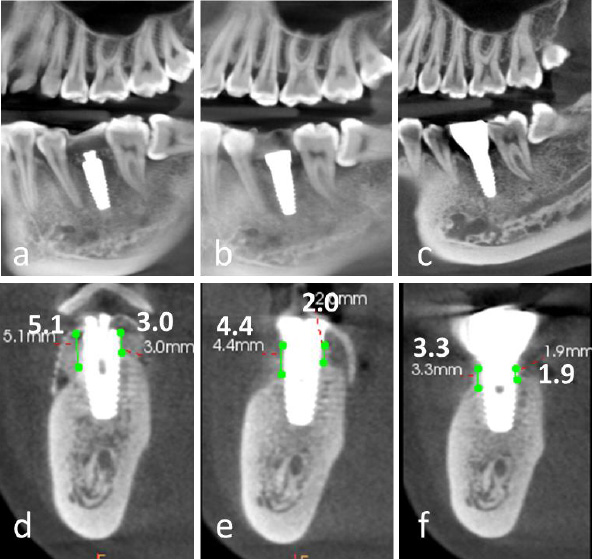
Comparative analysis of cone-beam computed tomography (CBCT) imaging at different treatment stages. (a-c) Panoramic reconstructed views: (a) Immediate postoperative evaluation, (b) Healing abutment placement phase, (c) 2.5-year follow-up after prosthetic delivery, (d-f) Cross-sectional views corresponding to respective treatment phases, (d) Postoperative implant positioning, (e) Peri-implant tissue maturation during healing phase, (f) Long-term (2.5-year) evaluation of peri-implant bone stability and prosthetic integration.
However, reconstructing severe vertical or horizontal bone defects with GBR remains a significant challenge due to the stiffness limitations of traditional barrier membranes [5]. Maintaining a suitable and stable bone regeneration space is challenging, and micromotion can easily occur, potentially affecting the blood supply [6, 8].
When the alveolar bone has severe vertical or horizontal bone defects, many clinical studies suggest that titanium mesh demonstrates superior mechanical properties and excellent osteogenic performance during application [7].
4.2. The Present Case Report: Exposure to Titanium Mesh
The present case report describes a vertical bone defect in the posterior mandibular region that underwent ridge augmentation and implant placement concurrently using a new technique involving GBR with a titanium mesh shelter fixed onto the dental implant via a new type of healing cap.
In most cases, the titanium mesh is positioned first, followed by the barrier membrane [8, 19, 20]. However, in this case, the barrier membrane was placed over the titanium mesh. Tension-free closure using resorbable suturing material could not be achieved due to insufficient soft tissue.
The biocompatibility of the absorbable collagen membrane can promote mucosal healing. However, a retrospective cohort study found no statistical difference in the exposure rate of GBR with titanium mesh with or without covering an absorbable collagen membrane [21, 22]. Even in cases where exposure did not occur, dense fibrous tissue was still found under the titanium mesh. Due to its high stiffness, low density, corrosion resistance, and good biocompatibility, the titanium mesh can protect the barrier membrane from premature absorption and loss of the biological barrier [23, 24].
Wound dehiscence is a common complication in the application of titanium mesh in GBR, with the incidence of titanium mesh exposure reported to be as high as 20%–30%, and the highest reported exposure rate is 66% [22, 25]. The stiffness of titanium mesh and its sharp edges, generated during cutting and bending, may cause detrimental stimulation of mucosal flaps, leading to mucosal rupture and subsequent mesh exposure [21].
In this case, the titanium mesh was continuously exposed in the mouth, making postoperative maintenance crucial. Antibiotic ointment and a periodontal pack were applied within the first two weeks after surgery to prevent the retention of food residue and secondary infection. Regular follow-up visits and wound care were key to preventing infection. In this case, normal saline was used as an irrigation solution every week after surgery to remove food residues and attachments around the titanium mesh.
CONCLUSION
The novel technique for vertical bone augmentation using “N-Shaped” titanium mesh is regarded as an improved GBR technique. Although the technique has the risk of titanium mesh exposure, it may offer an effective solution for vertical bone defects in the posterior mandibular region.
AUTHORS’ CONTRIBUTIONS
The authors confirm their contribution to the paper as follows: H.L.: Responsible for data collection; S.L.: Contributed to the methodology; C.C.S.: Conducted the investigation; C.W.: Drafted the manuscript. All authors reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| GBR | = Guided Bone Regeneration |
| e-PTFE | = Expanded Polytetrafluoroethylene |
| CBCT | = Cone-Beam Computed Tomography |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Joint Committee on Clinical Investigation of The Seventh Medical Center of PLA General Hospital (Beijing, China) (No. 2017-036).
HUMAN AND ANIMAL RIGHTS
All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committee and with the 1975 Declaration of Helsinki, as revised in 2013.
AVAILABILITY OF DATA AND MATERIALS
All the data and supporting information are provided within the article.
FUNDING
This study was supported by the Open project of the State Key Laboratory of Military Stomatology, China (Grant number 2020KB04)
ACKNOWLEDGEMENTS
Declared none.


