All published articles of this journal are available on ScienceDirect.
Efficacy of Sticky Bone with Repeated Injectable Platelet-rich Fibrin Application in the Management of Periodontal Intrabony Defects: Randomized Controlled Clinical Trial
Abstract
Background
This study aimed to compare and evaluate the clinical and radiographic outcomes of using a xenograft alone versus a sticky bone combined with repeated injections of injectable platelet-rich fibrin (I-PRF) for the treatment of intrabony defects (IBDs) in patients with stage III periodontitis. The investigation was conducted with a rigorous randomized prospective study design, ensuring the validity of the results.
Methods
This clinical and radiographic study included thirty patients with intrabony defects (IBDs). The defects were randomly assigned to one of two groups: Group I received xenograft alone, while Group II received a combination of injectable platelet-rich fibrin (I-PRF) and xenograft, along with repeated I-PRF injections. Clinical and radiographic assessments were performed at baseline, 6 months, and 9 months. The collected data were tabulated and analyzed using SPSS software, version 22.
Results
The test group, which received repeated injections of I-PRF with sticky bone, showed statistically significant improvements in IBD reduction at the individual site after six and nine months compared to the same periods in the control group.
Discussion
In this study, there is marked improvement of (IBD) in the clinical and radiographic parameters due to the novel approach of repeated (I-PRF) injections. The results of this study, along with existing literature, support the incorporation of this novel approach into clinical practice to enhance (IBD) treatment predictability.
Conclusion
This finding suggests that repeated injection of I-PRF with sticky bone may offer a more effective treatment option for managing IBDs than xenografts alone.
1. INTRODUCTION
Periodontal diseases have a complex etiology, primarily produced by unique periodontopathic bacteria and their metabolic byproducts [1]. A key objective of periodontal therapy is to restore the lost supporting periodontal tissues to their original condition. Histologically, healing after nonsurgical treatment and conventional surgical procedures is typically characterized by the formation of a long junctional epithelium along the root surfaces. As a result, reconstructive periodontal surgery is often required [2].
Although no regenerative therapy can ensure complete periodontal reconstruction, bone grafts, and biologics hold significant promise. Substantial clinical evidence demonstrates that grafts consistently result in greater bone fill compared to non-grafted controls [3-5]. Bone substitutes are increasingly recognized as viable alternatives to autogenous bone in ridge augmentation procedures, helping to reduce the morbidity associated with harvesting autogenous grafts and the limited volume typically available [6]. Xenograft materials serve as scaffolds or calcified matrices, supporting the proliferation of surrounding osteogenic cells while preserving space and stabilizing the blood clot [7]. Platelet-based preparations from patient blood are low-cost alternatives to commercially available bioactive materials. Activated platelets secrete various proteins and growth factors, such as platelet-derived growth factor (PDGF), transforming Growth Factor-2 (TGF-2), vascular Endothelial Growth Factor (VEGF), bone Morphogenetic Protein (BMPs), transforming growth factor-β (TGF-β), and insulin-like Growth Factor (IGF) [8].
These growth factors attract undifferentiated mesenchymal cells to the injury site and promote angiogenesis, chemotaxis, and cell proliferation. Additionally, they regulate the synthesis and degradation of extracellular matrix proteins, stimulate osteogenesis, and accelerate peri-implant wound healing and osseointegration [9].
Marx et al. pioneered the first type of platelet concentrate in the dental field using platelet-rich plasma (PRP) [10]. Since then, PRP has been widely applied across dentistry, orthopedics, and aesthetic medicine for tissue regeneration, primarily due to its ability to enhance angiogenesis [11].
Despite its promise, reports have highlighted limitations in the regenerative potential of earlier platelet concentrates, primarily due to the inclusion of anticoagulants, which are known as inhibitors of tissue regeneration, as well as challenges in their biochemical handling [10]. To address these issues, a new formulation known as platelet-rich fibrin (PRF) was introduced in 2001, specifically designed to eliminate the need for anticoagulants [12]. Because PRF is free of anticoagulants, it naturally forms a fibrin clot within minutes of blood collection. This clot serves as a scaffold in a three-dimensional network that supports tissue regeneration [13]. In addition to its complete immunobiocompatibility, PRF offers several other advantages, including enhanced angiogenesis that promotes faster wound healing. When used in combination with bone grafts, it may also function as a “biological connector.” For these reasons, PRF has gained widespread adoption in oral surgery, and its use continues to grow rapidly [14].
Remarkably, over 20 years have passed since Choukroun et al. introduced the concept of platelet concentrates without anticoagulants, and considerable advancements have been made. In recent years, notable efforts have focused on developing injectable platelet-rich fibrin (I-PRF), a liquid form of PRF designed to enhance biomaterial integration. I-PRF facilitates better mixing with other platelet concentrates and forms a fibrin network shortly after combining with bone graft materials or coatings, thereby improving the durability and stability of biomaterials during regenerative procedures. Although both forms of PRF demonstrated high compatibility in vitro, with elevated levels of cell viability, I-PRF significantly enhanced the migration, proliferation, adhesion, and spreading of human gingival fibroblasts. Furthermore, I-PRF promoted the release of wound-healing growth factors, such as PDGF and TGF-β, and stimulated collagen synthesis. The absence of anticoagulants and the natural formulation of I-PRF contributed to improved regenerative potential in the current in vitro studies [15]. Recently, an injectable PRF formulation was developed using a simplified centrifugation protocol: a single-step spin at 700 rpm (60 g) for 3 minutes. Due to the absence of anticoagulants, I-PRF must be used within 15 minutes before it polymerizes into a fibrin clot [16].
Due to the lower centrifugation speeds, Ghanaati et al. and Choukroun et al. found that injectable PRF (I-PRF) contains a higher concentration of cells, particularly leukocytes, prior to fibrin polymerization, when compared to other platelet concentrates [15, 17]. Although not all platelet concentrate formulations retain leukocytes, these immune cells play a crucial role in host defense against pathogens and contribute to wound healing by releasing various growth factors [18]. In earlier formulations, blood samples were collected in glass or plastic tubes with blue plastic caps and activated by silica, which initiated fibrin formation. However, some researchers have suggested that silica particles act merely as catalysts and may pose potential harm to patients, even when not directly incorporated into the final material [19]. To address these concerns and overcome limitations associated with leukocyte-rich PRF (L-PRF), a newer formulation known as titanium-prepared platelet-rich fibrin (T-PRF) was developed [20]. T-PRF features a denser and more compact fibrin matrix than L-PRF. This denser fibrin structure is essential for prolonging intra-tissue fibrin degradation and enabling a sustained, gradual release of growth factors over time [21, 22].
Sohn et al. utilized injectable PRF (I-PRF) for alveolar ridge reconstruction and sinus augmentation [23]. The preparation of I-PRF is based on the low-speed centrifugation concept, which enables the concentration of platelets and leukocytes while maintaining a continuous release of growth factors. In their 2015 study, Kim introduced the concept of “sticky bone,” a cohesive, fibrin-rich bone graft that acts as a protective layer over both the periosteum and alveolar bone. Sticky bone is moldable, easy to handle, and adheres well to bone defects [24]. It also reduces both micro- and macro-movement of the grafted material, promoting successful bone augmentation during healing without requiring guided bone regeneration (GBR) membranes or titanium meshes. Moreover, sticky bone eliminates the need for a separate barrier membrane, as its fibrin matrix stimulates platelets and leukocytes to release signaling molecules that significantly enhance the regeneration of hard and soft tissues. The interconnected fibrin network also prevents the invasion of soft tissue and epithelial cells into the graft site [24, 25]. However, PRF membranes typically have relatively short resorption times, lasting approximately 10 to 14 days, considerably shorter than the duration required for complete periodontal regeneration [26]. As a result, repeated injections of I-PRF at 14 and 28 days postoperatively may be necessary to prolong the activity of released growth factors during the healing phase, particularly in the intra-bony compartment, since prior studies primarily focused on effects in the soft tissue components [27].
2. MATERIALS AND METHODS
2.1. Ethical Consideration and Patient Recruitment
This prospective, controlled, randomized clinical study included thirty nonsmoking patients with stage III periodontitis [28, 29]. Participants, aged between 28 and 51 years (mean age 39.6 ± 3.9), were consecutively recruited from a pool of patients seeking periodontal treatment at the Department of Periodontics, College of Dental Medicine, Al-Azhar University, Cairo, Egypt.
Patients were selected based on the following inclusion criteria:1) no systemic disorders that may affect successful therapy outcomes; 2) satisfactory compliance with plaque control guidelines following initial phase therapy; 3) all teeth involved in the trial were vital and had no mobility; 4) each person supplied predominately two- or three-osseous wall intrabony interproximal defects adjacent to the premolar or molar teeth without furcation involvement; 5) selected intrabony defects (IBD) measured from the marginal bone level to the bottom of the defect on accurate digital periapical radiographs of ≥ 3 mm, demonstrating no cratering that involved both distal and mesial sides of adjacent teeth; 6) probing depth (PD) of 6 mm and clinical attachment loss (CAL) of 5 mm at the position of intraosseous defects within four weeks after starting cause-related therapy; 7) availability for monitoring, follow-up, and maintenance plan; and 8) patients did not receive any periodontal treatment in the preceding year. Exclusion criteria were: 1) furcation involvement, 2) smokers, and 3) Patients with debilitating systemic diseases or conditions that could significantly impair wound healing were excluded from the study.
The study protocol was reviewed and approved by Al-Azhar University. All patients were informed about the research procedures and provided written consent by signing the Al-Azhar University informed consent form. This clinical trial was registered under the following number: (748/2757). The study was conducted in accordance with the Declaration of Helsinki and received approval from the Institutional Review Board (IRB) of Al-Azhar University, Cairo, Egypt (997/341), for studies involving human subjects.
2.2. Sample Size Calculations
Sample size analysis was performed using G*Power software, version 3.1.9.4. The primary outcome measure was the difference in bone fill between the two groups. Based on a previous study by Mathur et al. (2015), an effect size of 1.26 was identified [30]. To achieve a power of 0.80 (80%) with an alpha level of 0.05 (5%), it was calculated that 15 patients would be required in each group. Therefore, a total of 30 patients were included in the study to ensure adequate statistical power to detect significant differences between the treatment modalities.
A total of 90 patients were assessed for eligibility. Of these, fifty were excluded, twenty-seven for not meeting the inclusion criteria, and twenty-three who declined to participate. Ultimately, 40 patients were enrolled and randomly assigned to two groups. During the study, seven patients were lost to follow-up, and three patients discontinued the intervention. However, data from all enrolled participants were included in the final analysis (Fig. 1).
2.3. Presurgical Therapy and Patient Grouping
Preliminary cause-related therapy included full-mouth supra, subgingival scaling, and root planning throughout all quadrants. This process was carried out by hand and ultrasonic instrumentation with a Piezoelectric-10 tip. Patients were recalled after 24 hours to complete their initial therapy and receive instructions for comprehensive mechanical plaque control.
A re-evaluation was conducted four weeks after the initial therapy to confirm the need for periodontal surgery. Surgery was indicated when an interproximal site exhibited a probing depth (PD) ≥ 6 mm, clinical attachment loss (CAL) ≥ 5 mm, and interproximal intrabony defects (IBD) ≥ 3 mm. Clinical assessments, including the plaque index (PI) [31], were used to establish the baseline periodontal condition of the selected sites.
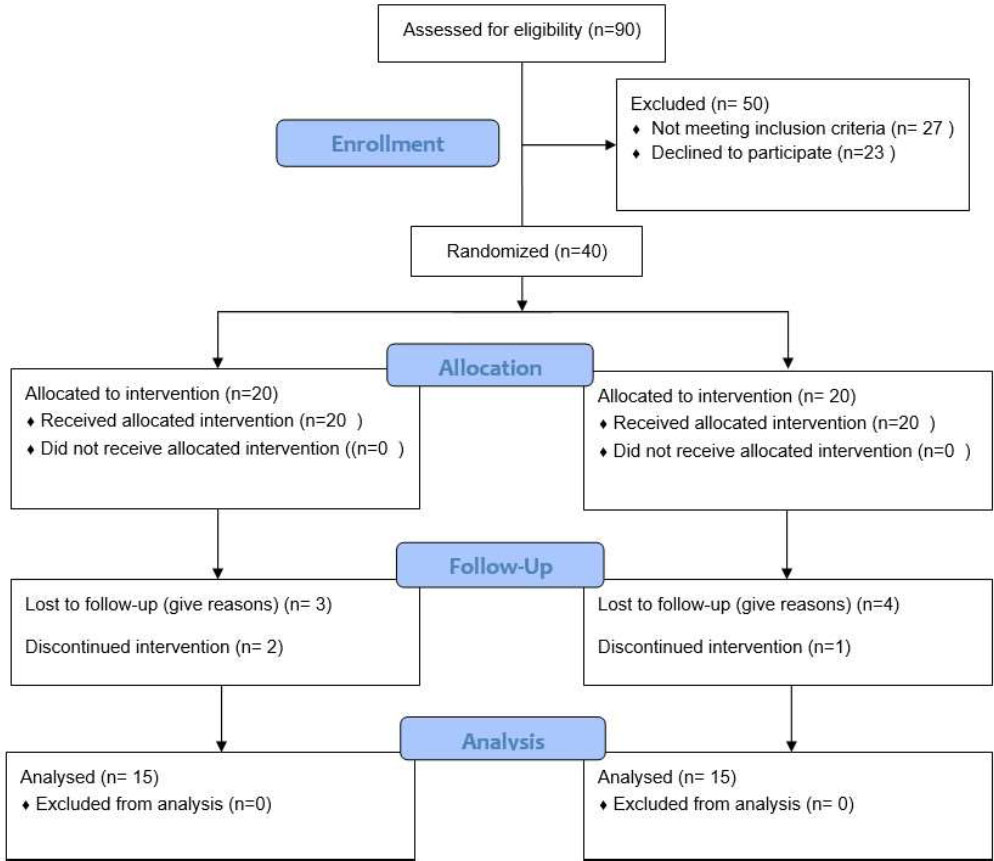
Flow diagram of patient recruitment and inclusion.
While an acrylic stent was in place, PD [32], CAL [33], and (IBD) were measured as follows: the corresponding defect base level (DBL) was determined by measuring from the CEJ to the defect's base, and the relative crestal bone level (CBL) was determined by measuring from the CEJ to the alveolar margin. Digital periapical radiographs were recorded utilizing intraoral size two dental films and holders that were guided in a standardized position attached to the position indicated device with the aid of an acrylic resin customized bite block with Rinn-XCP employing x-ray unit was operated at 70 kV, 9 mA, and an exposure time of 0.6-second, Radiographic images were analyzed using Romexis software, version 5.1.1.2 (2D module).
Preliminary cause-related therapy and clinical parameters were measured by an experienced calibrated examiner who was not otherwise engaged in the study (MTD). A calibration session was conducted twice, 48 hours apart, to assess intra-examiner repeatability. Calibration was allowed if 90% of the readings could be replicated within only one mm difference.
2.4. Randomization and Allocation Concealment
Patients were randomly assigned to one of two groups (15 patients per group): the Xenograft Control Group (Gr1) and the I-PRF Combined Xenograft Group (Gr2). The second group received a combination treatment consisting of injectable platelet-rich fibrin (I-PRF) and xenograft material. To optimize regenerative outcomes, I-PRF injections were repeated on days 14 and 28. Randomization was performed immediately before surgery using an online web-based tool (http://www.randomizer.org). Each participant was assigned a unique identification code. Randomization and allocation concealment were carried out by an independent faculty member who was blinded to the study objectives. Participants were instructed not to disclose their group assignments to the treatment therapist.
2.5. Surgical Procedures
Following blood collection, two 10 mL silica-coated plastic tubes (blue-capped, without anticoagulant) containing whole blood were centrifuged at 700 rpm (60 g) for 3 minutes at room temperature using a Heraeus Megafuge™ 16R centrifuge (Thermo Scientific, USA). The upper liquid layer, representing the injectable platelet-rich fibrin (I-PRF), was collected and mixed with the xenograft material to form the moldable compound known as sticky bone.
2.6. Intervention
The surgical procedure commenced only when patients exhibited a full-mouth plaque score of less than 1 and a plaque score of 0 at the surgical site. All procedures were performed by an experienced periodontist with over 17 years of clinical practice. Following mucoperiosteal flap reflection on the affected tooth and adjacent areas, granulation tissue was carefully debrided from the intrabony defects using Gracey metal curettes (11/12, Hu-Friedy, Chicago, IL, USA). Root surfaces were thoroughly scaled and planed using a combination of ultrasonic and hand instruments. In Group 1, a bovine-derived xenograft (Tutogen–RTI Biologics, Neunkirchen am Brand, Germany) was gently condensed into the intrabony defects using light pressure (Fig. 2). In Group 2, the defects were filled with xenograft material mixed with injectable platelet-rich fibrin (I-PRF). The flap was then repositioned and secured using internal horizontal and vertical mattress sutures. I-PRF was administered through repeated microinjections into the gingival sulcus using a microneedle (0.25 mm (31G) × 5 mm, BD Glide™ insulin syringe) until gingival blanching and fullness were observed. Gentle pressure with moist gauze was applied to the injection site for five minutes following delivery. I-PRF injections were repeated on days 14 and 28 postoperatively (Fig. 3).
2.7. Postoperative Recommendations
Postoperative instructions were provided to each participant both verbally and in written form. Patients were prescribed amoxicillin (500 mg) to be taken every eight hours for five days, along with diclofenac sodium (50 mg), administered three times daily for the same duration. To support plaque control, patients were instructed to rinse with 0.12% chlorhexidine hydrochloride (Surgident Co. Ltd., Daegu, Korea) for one minute, twice daily, for two weeks. During this period, they were advised to avoid brushing or cleaning teeth in the surgical areas. The sutures were removed two weeks after surgery. Follow-up visits were scheduled every two weeks during the first two months postoperatively to monitor adverse tissue reactions and to reinforce oral hygiene practices.
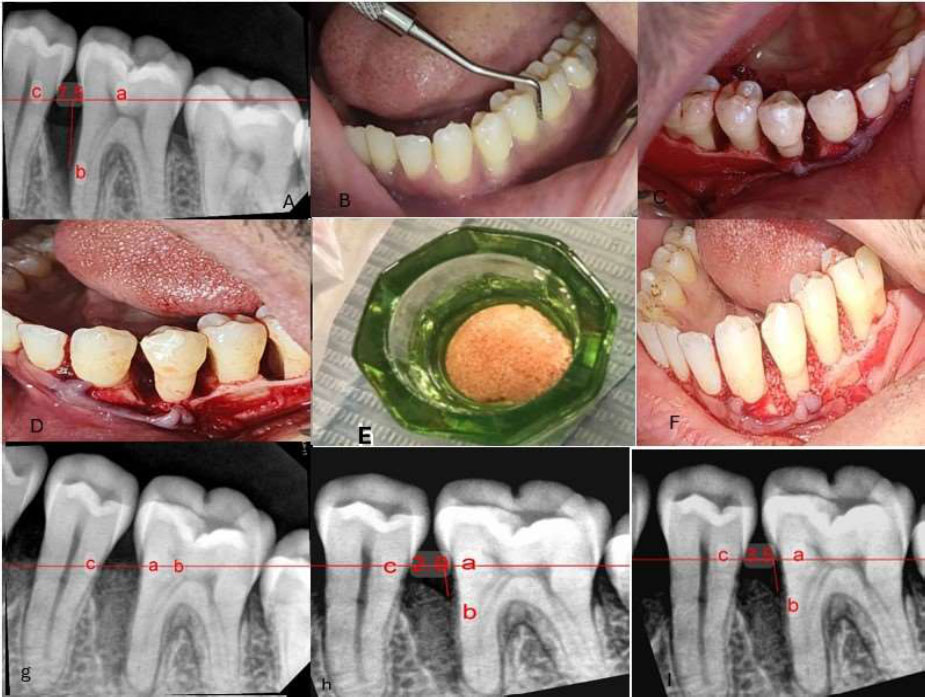
(a) Preoperative radiograph showing the intraosseous defect. (b) Presurgical assessment of probing depth using William’s probe. (c, d) Open flap reflection and debridement. (e) Preparation of the xenograft material. (f) Application of the bone graft into the defect. (g) Immediate postoperative radiograph. (h) Six-month postoperative radiograph. (i) Nine-month postoperative radiograph. (a- cementoenamel junction b- the base of defect c- crest of bone defect).
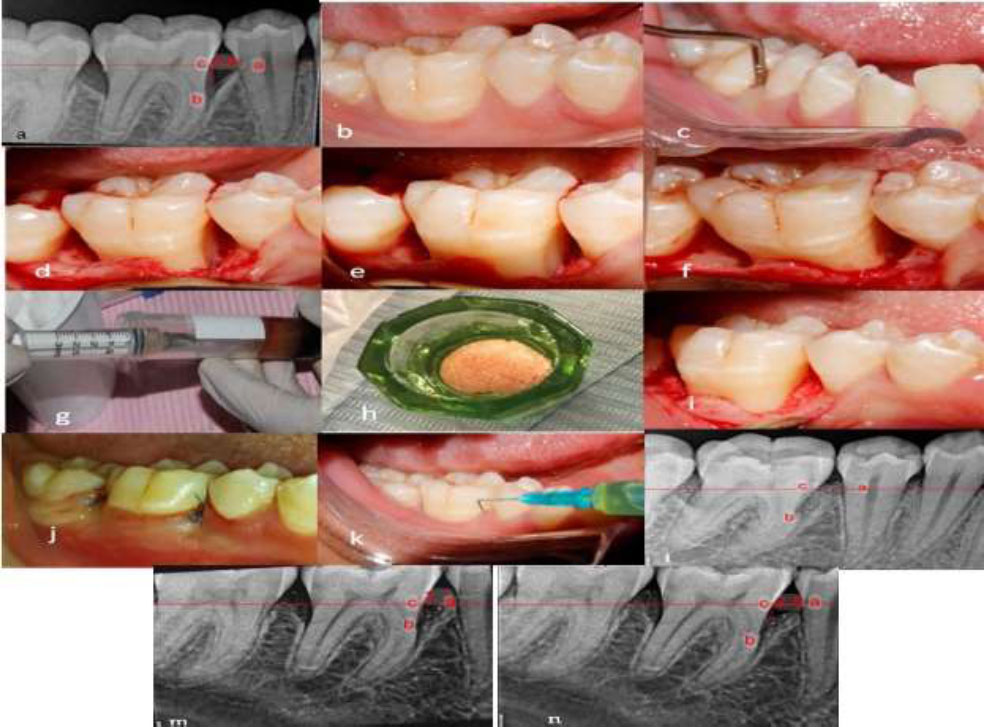
(a) Preoperative radiograph showing the intraosseous defect. (b) Preoperative clinical view. (c) Presurgical assessment of probing depth using William’s probe. (d–f) Open flap reflection and debridement. (g) Preparation of I-PRF. (h) Preparation of sticky bone. (i) Application of the bone graft into the defect. (j) Immediate postoperative clinical view. (k) Injection of I-PRF into the gingival sulcus. (l) Immediate postoperative radiograph. (m) Six-month postoperative radiograph. (n) Nine-month postoperative radiograph. (a- cementoenamel junction b- the base of defect c- the crest of bone defect).
2.8. Follow-up and Re-evaluation
Three weeks after surgery, all patients were instructed to resume standard mechanical oral hygiene practices, including brushing with a soft toothbrush using the rolling technique and flossing. During each recall visit, supportive periodontal care was provided, which included reinforcement of oral hygiene instructions, professional dental cleaning, and supragingival scaling.
3. RESULTS
3.1. Statistical Analysis
The data was loaded into the computer and analyzed with IBM SPSS software edition 22.0, IBM Corporation, Armonk, New York. Periodontal condition information is presented as mean ± SD values. p-values were calculated for the one-tailed hypothesis (two-independent samples t-test).
This randomized, controlled clinical study included thirty patients diagnosed with stage III periodontitis. Periodontal intrabony defects were randomly assigned to one of two treatment groups: Group I received xenograft alone, while Group II received a combination of injectable platelet-rich fibrin (I-PRF) and xenograft, along with repeated I-PRF injections.
The treated intrabony defects were distributed as follows: for the (Gr- 1 sites), eight two-wall and seven three-wall defects; for the (Gr- 2 sites), ten two-wall and five three-wall defects.
All patient's uneventful healing was noticed. Regarding the Plaque index, there was a statistically non-significant difference among the groups at baseline, 6 months, and 9 months, respectively (Table 1). PPD and CAL showed statistically significant improvements between the groups from baseline to six and nine months (Table 2). For IBD, there was no statistically significant difference between the groups at baseline (4.04 ± 0.29 for Group I; 4.00 ± 0.28 for Group II). At six months, IBD values were 2.16 ± 0.44 for Group I and 1.82 ± 0.07 for Group II, showing a statistically significant difference between the groups. At nine months, IBD was 2.12 ± 0.45 for Group I and 1.72 ± 0.12 for Group II, with a statistically significant difference also observed at this time point (Table 3).
| - | - | Baseline | 6 Months | 9 Months | p-value | 6 Months/ Baseline |
9 Months/ Baseline |
9 Months/ 6 Months |
|---|---|---|---|---|---|---|---|---|
| PI | Group1 | 0.55±0.0 | 0.55±0.3 | 0.57±0.4 | 0.1 | 1.04±0.84 | 1.39±0.71 | 1.16±0.31 |
| Group2 | 0.54±0.1 | 0.55±0.2 | 0.56±0.3 | 0.7 | 0.97±0.46 | 0.96±0.53 | 1.18±0.68 | |
| PD | Group 1 | 6.28±0.26 | 3.78±0.19 | 3.66±0.25 | <0.001 | 0.60±0.04 | 0.58±0.04 | 0.97±0.08 |
| Group 2 | 6.15±0.23 | 3.19±0.1 | 2.93±0.06 | <0.001 | 0.51±0.02 | 0.47±0.02 | 0.78±0.05 | |
| CAL | Group1 | 4.28±0.26 | 2.58±0.36 | 2.50±0.37 | <0.001 | 0.60±0.09 | 0.58±0.08 | 0.98±0.17 |
| Group2 | 4.31±0.28 | 2.34±0.12 | 2.09±0.07 | <0.001 | 0.54±0.04 | 0.48±0.03 | 0.82±0.11 | |
| IBD | Group1 | 4.04±0.29 | 2.16±0.44 | 2.12±0.45 | <0.001 | 0.53±0.13 | 0.52±0.13 | 1.01±0.29 |
| Group2 | 4.00±0.28 | 1.82#±0.07 | 1.72#±0.12 | <0.001 | 0.45±0.04 | 0.42±0.04 | 0.83±0.17 |
| - | - | Group 1 | Group 2 | p-value |
|---|---|---|---|---|
| PI | Baseline | 0.55±0.1 | 0.54±0.1 | 0.49 |
| 6 Months | 0.55±0.3 | 0.55±0.2 | 0.93 | |
| 9 Months | 0.57±0.4 | 0.56±0.3 | 0.11 | |
| PD | Baseline | 6.28±0.26 | 6.15±0.23 | 0.07 |
| 6 Months | 3.78±0.19 | 3.19#±0.1 | ≤0.001 | |
| 9 Months | 3.66±0.25 | 2.93±0.06 | ≤0.001 | |
| CAL | Baseline | 4.28±0.26 | 4.31±0.28 | 0.46 |
| 6 Months | 2.58±0.36 | 2.34±0.12 | 0.003 | |
| 9 Months | 2.50±0.37 | 2.09±0.07 | ≤0.001 | |
| IBD | Baseline | 4.04±0.29 | 4.00±0.28 | 0.35 |
| 6 Months | 2.16±0.44 | 1.82#±0.07 | <0.001 | |
| 9 Months | 2.12±0.45 | 1.72#±0.12 | <0.001 |
| IBD | Baseline | 9 Months | P-value | Difference | Percentage of Change |
|---|---|---|---|---|---|
| Group I (n = 15) | 4.04±0.29 | 2.12±0.45 | <0.001 | 1.85±0.57 | 45.18±14.03 |
| Group II (n = 15) | 4.00±0.28 | 1.72±0.12 | <0.001* | 2.29±0.31 | 55.75±7.7 |
| p-value | 0.35 | <0.001 | * | - | - |
4. DISCUSSION
Periodontitis is a multifactorial chronic inflammatory disease associated with dysbiotic plaque biofilms and characterized by progressive destruction of the tooth-supporting apparatus. Periodontal reconstructive techniques have progressed from debriding angular bony defects and bone swaging to using diverse materials in periodontal defect regeneration [36]. The explosion of information and awareness of the importance of platelet concentrates, their ways of action, and molecular signal pathways have paved the way for a plethora of new therapeutic options that can be used for in situ regeneration of periodontium.
The aim of this investigation was to compare the effectiveness of a bovine-derived xenograft alone (control group) with that of a xenograft combined with injectable platelet-rich fibrin (I-PRF), known as sticky bone, followed by repeated postoperative I-PRF injections for the treatment of intrabony defects (IBD) in patients with stage III periodontitis. This clinical study demonstrated that the use of repeated I-PRF injections in combination with xenografts significantly improved both clinical and radiographic outcomes compared to xenografts alone. The liquid form of PRF used in this study was consistent with the injectable PRF technique first described in 2017 [37].
Autologous platelet concentrate has been widely employed in dentistry and medicine, notably PRF, which has become an integral component of treatment regimens in periodontal plastic surgery, oral and maxillofacial surgery, and implant placement. PRF has considerable advantages for soft tissue reconstruction, wound repair, and bone regeneration. PRF is regularly available based on consistency as a membrane and gel; however, it cannot be infused or injected. A minor adjustment in the patient's blood-spinning regimen with lower speed and less time led to the invention of injectable PRF, a liquid version of PRF [38].
Miron et al. investigated a liquid formulation of platelet concentrates known as I-PRF, which does not require anticoagulants [37]. Injectable-PRF (centrifuged at 700 rpm. (60G) for 3 minutes) was tested for growth factor production, which lasted for ten days (8 donor samples). PRF can increase fibroblast attraction, provide high levels of several growth factors, and express fibroblast growth factor, collagen type 1, and transforming growth factor (TGF- β). More research is needed to confirm the therapeutic value of I-PRF as a bioactive compound capable of improving and stimulating tissue regeneration.
Previously, PRF was sliced into tiny pieces before being mixed with the bone substitutes [39-41]. These additional procedures are complicated and lengthen the entire regenerative surgical operation, which is undesirable. Because alveolar bone exposure to air causes bone resorption, eliminating the need to slice PRF reduces the time of alveolar bone exposure [42]. In this investigation, we used sticky bone and xenograft without barrier membranes. According to Zang. F.'s meta-analysis, there is no significant difference in clinical effectiveness between basic bone grafting and bone grafting mixed with membrane materials in periodontal regeneration [43].
PPD and CAL improved statistically significantly in each group from baseline to 6 and 9 months. These findings are comparable with those of Patel et al., who compared open flap debridement (OFD) combined with and without PRF utilization in intrabony defects. The study reported mean values for PPD reduction of 3.0 ± 1.70 in the test group and 1.11 ± 0.45 in the control group, and CAL gain of 3.20 ± 1.14 and 0.90 ± 0.32 in the test and control groups, respectively [44].
Vuckovic et al. reported improvements in CAL gain and PPD reduction in the non-surgical management of periodontitis, both with and without the use of I-PRF. The study reported a greater CAL gain in the I-PRF group (0.9 mm) compared to the non-I-PRF group (0.33 mm), and a greater reduction in PPD with mean values of 1.95 mm in the test group versus 1.37 mm in the control group, both favoring the test group [45].
Additionally, Liu et al. demonstrated that 12 months after the implantation of xenograft bovine porous type GTR in combination with and without PRF in intrabony defects, there was a statistically significant variance in PD reduction and CAL gain between the two groups, favoring the test group [46]. The two groups had a 0.6 to 0.7 mm difference in PD and a 0.9 to 1.1 mm difference in CAL gain [46]. When Vu and Pham evaluated treating intrabony deficiencies with OFD alone versus adding PRF, they found this was also true. After six months, they observed a statistically significant difference between the test and the control groups in mean PD decrease (3.30±0.84; 2.57±1.36) and CAL gain (3.33±0.71; 2.23±1.22), respectively [47].
For IBDs, the results of defect reduction were statistically significant for both groups, with Group II showing superior results. Each group had a statistically significant reduction in IBD components from baseline to 9 months postoperatively. The mean and SD for bone fill were significantly different between the two groups (2.12±0.45; 1.72±0.12), and the percentage of change of defect reduction as follows (45.18±14.03, 55.75±7.7) for control and test group respectively. Patel et al. found that, after the study period, the PRF group showed a significantly higher percentage of intrabony component fill compared to the control group (45.18% vs. 21.6%) [44].
These findings contrast Taalab and Melek's fidings, which examined IBD reduction 6 months after β-tri-calcium phosphate graft application and an absorbable membrane combined with and without I-PRF [48]. The test and control groups had similar mean bone growth (4.6±0.44; 3±0.4), with no significant difference between them. This is consistent with the outcomes of Liu et al., who discovered a non-statistically significant difference (0.5%) in the change in IBD after 12 months between both groups [46]. Repeated injections were administered through the upper 2 mm of the gingival sulcus to minimize tissue injury and avoid disrupting the healing process.
A plausible explanation for the enhanced regeneration observed in the test group is that repeated I-PRF injections helped sustain its biological activity over time.: 1) since I-PRF provides a good supply of autologous growth factors [49]. That may stimulate the attraction, proliferation, and development of many cells of periodontium, thereby boosting periodontal regeneration [50, 51]. 2) I-PRF possesses antibacterial properties against a variety of periodontal pathogenic microbiota, particularly P. gingivalis. Furthermore, injectable PRF is more effective than standard PRF against P. gingivalis [52]. 3) I-PRF suppresses osteoclast formation and osteoclastogenesis [53]. 4) Injectable PRF has an anti-inflammatory impact by inhibiting macrophage and dendritic cell immune responses [52]. In this study, there is a marked improvement of intrabony defect (IBD) in the clinical and radiographic parameters due to repeated platelet-rich fibrin (I-PRF) injections through the continuous release of growth factor throughout the initial phase of healing after periodontal surgery. The results shed light on this novel treatment protocol. Therefore, this article can emphasize the feasibility of the clinical applications of this novel treatment protocol
4.1. limitation
This study has several limitations. First, the follow-up period was relatively short, although previous multicenter clinical trials have also used a 9-month follow-up period [34]. Second, bone width was not assessed; the study focused solely on measuring the depth of angular bone defects. Third, patient morbidity associated with repeated blood withdrawal for I-PRF preparation should be considered. Future longitudinal clinical and histological studies with larger sample sizes are warranted to further evaluate the regenerative potential of repeated I-PRF injections in combination with sticky bone and to confirm its efficacy in the treatment of intrabony periodontal defects.
CONCLUSION
Based on the findings of this study, repeated I-PRF injections on days 14 and 28 postoperatively led to significant improvements in both clinical and radiographic parameters. This effect is likely attributed to the sustained release of growth factors during the initial healing phase following periodontal surgery, reinforcing the potential of this novel treatment protocol. The liquid form of I-PRF makes it particularly suitable for periodontal pocket applications. Its repeated administration demonstrated superior regenerative outcomes within the first six months, particularly in terms of original defect resolution and defect fill, highlighting the enhanced regenerative capacity of I-PRF when applied in a staged manner.
AUTHORS’ CONTRIBUTIONS
The authors confirm contribution to the paper as follows: A.M.B.: Study, concept and design; A.K.A.: Writing the paper; I.S.S., A.H.H., H.M.A.: Methodology; M.M.H.: Writing original draft; A.G.B., M.T.D.: Investigation; M.H.S.: Data curation; A.M.S.: Validation. All authors have read and agreed to the published version of the manuscript.
LIST OF ABBREVIATIONS
| IBDs | = Intrabony Defects |
| I-PRF | = Injectable Platelet-rich Fibrin |
| PDGF | = Platelet-derived Growth Factor |
| TGF-2 | = Transforming Growth Factor-2 |
| VEGF | = Endothelial Growth Factor |
| BMPs | = Bone Morphogenetic Protein |
| TGF-β | = Transforming Growth Factor-β |
| IGF | = Insulin-like Growth Factor |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Institutional Review Board of Al-Azhar University, Cairo, Egypt (997/341).
HUMAN AND ANIMAL RIGHTS
All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committees and with the 1975 Declaration of Helsinki, as revised in 2013.
AVAILABILITY OF DATA AND MATERIALS
The authors confirm that the data supporting the findings of this research are available within the article.
FUNDING
The authors would like to thank Deanship of Scientific Research at Majmaah University for supporting this work. Name of the funding agency: Majmaah University, Funder ID: 11952, Awards/Grant number/Project Number: R-2025-1807.
ACKNOWLEDGEMENTS
Declared none.


