All published articles of this journal are available on ScienceDirect.
The Potential Ameliorative Effects of Flaxseeds on Methotrexate-induced Oxidative and Inflammatory Changes in the Tongue of Albino Rats
Abstract
Introduction
Oral mucositis is one of the significant complications of methotrexate. It is a form of stomatitis, which refers to ulceration and inflammation of the mucosal tissue.
Methods
In this study, 30 male albino rats were included, and they were divided into 3 groups. Group I received 0.6 ml distilled water for 8 consecutive days, with an injection of 0.5 ml saline on day 4. Group II received 0.6 ml distilled water for 8 consecutive days, with an injection of 60 mg/kg b.w of methotrexate on day 4. In group III, 200mg/kg/mL of flaxseeds dissolved in 0.6 ml distilled water were administered for 8 consecutive days, with an injection of 60 mg/kg b.w of methotrexate on day 4. At the end of the 8th day, rats were immolated, and their tongues were dissected and processed for inspection by H&E stain, CD3, and PCNA.
Results
Histological and immunohistochemical results of group II disclosed pronounced deteriorative alternations in the tongues of rats, whereas, in group III, renewal of tongue tissue was observed after treatment with flaxseeds. Moreover, the statistical analysis revealed high immunoreactivity of CD3 in the methotrexate group compared to the control and flax seeds group and low immunoreactivity of PCNA in the methotrexate group compared to the control and flax seeds group.
Discussion
Considering the previous results, the study demonstrated the anti-inflammatory and anti-mucositis effects of flaxseeds on the lingual mucosa of MTX-treated rats.
Conclusion
Flaxseeds exhibited a reduction in histopathological impairment associated with methotrexate administration in the tongue tissues, with accompanying anti-inflammatory and antioxidant activities that can overcome the deleterious effect of methotrexate on mucosa.
1. INTRODUCTION
Methotrexate (MTX) is one of the most established chemotherapeutic agents. It is an antimetabolite and immune-regulating drug. When administered in high doses, it produces a chemotherapeutic response and is used for the treatment of cancer. Notwithstanding, when given in low doses, it can be effective for the treatment of rheumatic diseases [1]. MTX is a folic acid antagonist, which is a pivotal cofactor for DNA synthesis and cell turnover. This mechanism underlies its chemotherapeutic action but also contributes to the development of oral mucositis as a complication [2].
Although MTX has a crucial function in the treatment of several diseases, it has been found to cause gastrointestinal injury, nephrotoxicity, dermatologic toxicity, and hepatotoxicity [3]. These complications could be attributed to the diminution of folic acid that leads to metabolic modifications affecting the purine and pyrimidine metabolism [4]. Moreover, MTX could impede folic acid reductase, which can lead to the development of oral mucositis by interfering with DNA synthesis. This interference slows the regeneration capability of the epidermal cells in the stratum basale and hinders the proliferation of the mucous membrane [5]. It was found that MTX causes kidney malfunction either due to the precipitation of MTX and its metabolites in the renal tubules [6] or directly by the poisonous effect of MTX on the uriniferous tubules [7]. Thereafter, MTX is primarily (˃ 90%) flushed by the kidneys. MTX-induced end-stage kidney disease could hinder its eradication and consequently advance its plasma level [8]. MTX may raise the Glomerular Filtration Rate (GFR) and cause uremia and hematuria. Furthermore, acute tubular necrosis can also be caused due to high doses of MTX [9].
One of the important complications of MTX is oral mucositis. Oral toxicities, for instance, mucositis and stomatitis, are some of the critical complications related to cancer treatment [10]. Interleukin (IL)-1, Tumor Necrosis Factor (TNF), and oxygen radicals play a pivotal role in generating mucositis [11]. Matrix metallopeptidases play a role in tissue repair by regulating the homeostasis of the intercellular matrix and mediating the response to inflammation and tissue destruction during mucositis. Matrix metallopeptidases have crucial enzymes that regulate mucosal homeostasis. Previously, it was stated that proinflammatory cytokines contribute to the ulceration of the oral mucous membrane by disrupting the cell growth patterns and division rates of cells, primarily through the induction of apoptosis [12].
Generally, the oral stomatitis manifestations include pain, discomfort, and food sensitivity, which, in consequence, influence the nutritive level and patient’s capacity to bear chemotherapy. This sequentially influences cancer treatment as well patient’s subjective well-being [13].
Flaxseed (linseed, Linum usitatissimum, Linaceae) is a seed from the flax plant (Linum usitatissimum L.) and is extensively utilized as a natural product worldwide due to its benefits associated with its bioactive ingredients [14]. It is rich in macronutrients, dietary fiber, and lignans and is commonly used in food and health supplements [15]. Flaxseed oil is a rich source of omega-3 fatty acids, including α-Linolenic Acid (ALA), Eicosapentaenoic Acid (EPA), and Docosahexaenoic Acid (DHA), as well as omega-6 fatty acids, such as linoleic Acid (LA), Arachidonic Acid (AA), and Gamma-Linolenic Acid (GLA). It also contains lignans (secoisolariciresinol diglycoside, SDG), soluble anti-oxidants, and insoluble fibers [14].
Recently, it has been discovered that flaxseed can regulate the intestinal microbiome and enhance defense mechanisms against various diseases. The consumption of flaxseed augments the growth of beneficial microorganisms in the gut and also generates metabolites that play a significant role in lipid and glucose metabolism, as well as in maintaining metabolic balance [15]. Flaxseed oil and its fractions have predominant medicinal effects, including antioxidant, anti-inflammation, antiviral, and antibacterial properties [16]. In addition, they can also reduce blood glucose and cholesterol levels [17].
Consumption of flaxseed in regular food may diminish the risk of heart and blood vessel diseases, for instance, ischemic heart disease and stroke. Additionally, there is evidence that flaxseed has antineoplastic effects in cases of breast, prostate, and colon cancers. Linseed or flax is utilized industrially for the fabrication of linen cloth and in papermaking. The remnants after hydraulic fracturing contain about 35-40% protein and 3-4% oil; thus, they can be used as a source of feed for farm animals [18].
Taking into consideration the mentioned favorable effects of flaxseed, the present study was conducted to explore its probable protective effect on methotrexate-induced inflammation in the tongue of rats.
2. MATERIALS AND METHODS
2.1. Experimental Study
2.1.1. Experimental Animals
All research steps were conducted in accordance with the Mansoura University Animal Care and Use Committee, Egypt (approval No. MU-ACUC (DENT.R.24.12.18)). This study was carried out in adherence with the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines and regulations.
Thirty adult male albino rats aged 6 months, weighing ~200g, were utilized in the present research. Animals of each group were obtained from the animal house of the Faculty of Pharmacy, Mansoura University, and accommodated in discrete stainless steel aerated cages. Rats were housed and handled appropriately, fed an equity diet, and kept in secure, proper conditions. The total sample size was 30, with an N value of 10.
The sample size and N value were calculated according to certain factors, such as the acceptable level of significance (p-value), power (1 − β) of the study, expected 'clinically relevant' effect size, and underlying event rate in the population.
2.1.2. Drugs and Chemicals
Methotrexate vials 50 mg/2ml, under the trade name of Unitrexate, were obtained from HIKMA Pharmaceuticals, Cairo, Egypt. Flaxseeds were obtained from the agricultural seeds, herbs, and medical plants company in Cairo, Egypt.
2.1.3. Experimental Design
A presumptive analysis was carried out before conducting the experiment and prior to the delineation and planning stage of the experiment to determine the required sample size (N). This analysis helps in calculating the effect size, desired α level, and power level (1-β).
Animals were randomly and equally allocated into 3 groups as follows:
2.1.3.1. Group I (control group)
A total of 10 rats were administered 0.6 ml of distilled water by oral gavage for 8 successive days, with an intraperitoneal injection of 0.5 ml saline on day 4 of the experiment [19].
2.1.3.2. Group II
A total of 10 rats were administered 0.6 ml distilled water by oral gavage for 8 successive days [1], with an intraperitoneal injection of 60 mg/kg body weight of methotrexate on day 4 of the experiment [20].
2.1.3.3. Group III
A total of 10 rats were administered 200mg/kg/mL of flaxseeds [21] dissolved in 0.6 ml distilled water by oral gavage for 8 successive days, with an intraperitoneal injection of 60 mg/kg body weight of methotrexate on day 4 of the experiment [20].
At the end of the 8th day, rats were sacrificed by anesthesia overdose (ketamine 150 mg/kg b.w and xylazine 15 mg/kg b.w) [22], then their tongues were dissected and prepared for examination with Hematoxylin and Eosin stain (H&E), CD3, and Proliferating Cell Nuclear Antigen (PCNA).
2.2. Inclusion Criteria
The selection of animal models for the experiment was based on the following considerations: (1) usefulness as analogs, (2) translatability of findings, (3) genetic homogeneity, where applicable, (4) existing knowledge of their biological properties, and (5) cost and availability.
2.3. Exclusion Criteria
The animals were ruled out if impediment was expected during tongue cutting up or there was a failure to reach quality control excellence, such as unsatisfactory levels of pollutants or mediocre histological quality.
2.4. Histological Procedures
Hematoxylin and eosin staining was employed as a standard method for microscopic evaluation. Tongues were immersed in 10% formaldehyde for a day. Paraffin blocks were prepared, and 5 μm-thick tissue slices were cut and stained with H&E. These histological sections were examined by an optical light microscope.
2.5. Immunohistochemical Processing
2.5.1. CD3
Slides were dewaxed, humidified, washed in piped water, and rinsed for 5 minutes in dH2O. Then, antigen retrieval was subsequently carried out in Tris-EDTA buffer (pH 9.0, containing 10 mM Tris Base, 1mM EDTA, 0.05% tween 20) for 3 minutes using a Biocare decloaker (Biocare Medical, Concord, CA). Afterward, the slides were cooled for 30 minutes and rinsed in PBS. A serum block was performed for one hour at room temperature using 10% goat serum in PBS. Later on, an anti-CD3 antibody was incubated at 1:50 dilution in PBS containing 1% goat serum overnight at 4ºC. The slides were then rinsed for 5 min each in PBS thrice. Subsequently, Alexa Fluor 647 goat anti-rabbit (Invitrogen) was applied at a 1:1000 dilution in PBS for 30 minutes at room temperature in the dark. The slides were then rinsed for 5 min in PBS thrice. Afterward, the slides were gently counterstained by hematoxylin [23].
2.5.2. PCNA
Endogenous peroxidase blockage was performed using H2O2, and antigens were recovered by boiling in citrate buffer. Slides were then incubated with primary antibodies for Proliferating Cell Nuclear Antibody (PCNA monoclonal antibody, cat. #307901, Biolegend, California, USA) and interleukin-6 (IL-6 polyclonal antibody, cat. #A0286, ABclonal, Massachusetts, USA), and then incubated with the secondary biotinylated antibody and streptavidin-biotin complex. Afterward, diaminobenzidine chromogen (DAB kit, cat. # ab64238, Abcam, Cambridge, UK) was applied, followed by counterstaining with Harris hematoxylin [24].
2.6. Statistical Analysis
Interpretation of data was performed using SPSS software (SPSS, Inc., Chicago, IL, USA). Data were displayed in mean and standard deviation. One-way Analysis of Variance (ANOVA) and post-hoc Tukey statistical tests were carried out to measure the numeric values of the three groups. p-value ˂ 0.05 was considered statistically fundamental.
3. RESULTS
3.1. Hematoxylin and Eosin Staining Results
In Group I, the dorsal surface of the tongue showed a uniform arrangement of conical, filiform papillae, each covered by a thick keratin layer. The fungiform papillae appeared normal, each containing a single taste bud on its superior surface and covered by a thin keratinized layer. The epithelial rete ridges were long and uniform. The lamina propria consisted of collagen fibers and scattered fibroblasts (Fig. 1A-D).
In group II, the mucosal thickness was obviously thinner than that of the control group. The sections disclosed a non-uniform, thin, keratinized surface with irregular rete ridges. There were localized regions of disruption in gustatory cells, along with some transitional epithelium and connective tissue cells (Fig. 1B-E).
In group III, the tongue dorsum preserved its normal thickness and appearance. Regeneration of tongue papillae, normal lamina propria, and blood vessels was observed with the formation of the connective tissue (Fig. 1C-F).
3.2. Immunohistochemical Staining Results
3.2.1. CD3 Immunostaining Results
The positive nuclear staining appeared as a brown accumulation in the lamina propria. CD3, essentially found in T-lymphocytes, significantly increases during inflammation. Consequently, the number of CD3-positive cells per unit area was notably elevated in group II (Figs. 2A-C, 3, and Table 1).
3.2.2. PCNA Immunostaining Results
The anti-PCNA immunostaining revealed brown nuclear deposits. PCNA was expressed within epithelial basal and parabasal cell layers. PCNA labeling index was roughly calculated (the number of PCNA-positive cells divided by the total cell number in the basal layer). As PCNA is a marker of cellular proliferation, its reaction was mild in group II in comparison to groups I and III (Figs. 4A-C, 5, and Table 2).
4. DISCUSSION
Oral mucositis is an inflammatory state that occurs as a result of damage to oral mucosa following cytotoxic cancer chemotherapy inclusive of methotrexate. MTX is used to treat a range of adult and childhood cancers, such as central nervous system lymphoma, mantle cell lymphoma, and osteosarcoma [25]. The seriousness and period of mucositis are based on the dose and type of the drug used; nevertheless, in general, it could compromise the patient's nutritional status and overall health, potentially leading to a reduction or delay in chemotherapy dosing due to severe pain or other associated symptoms [26].
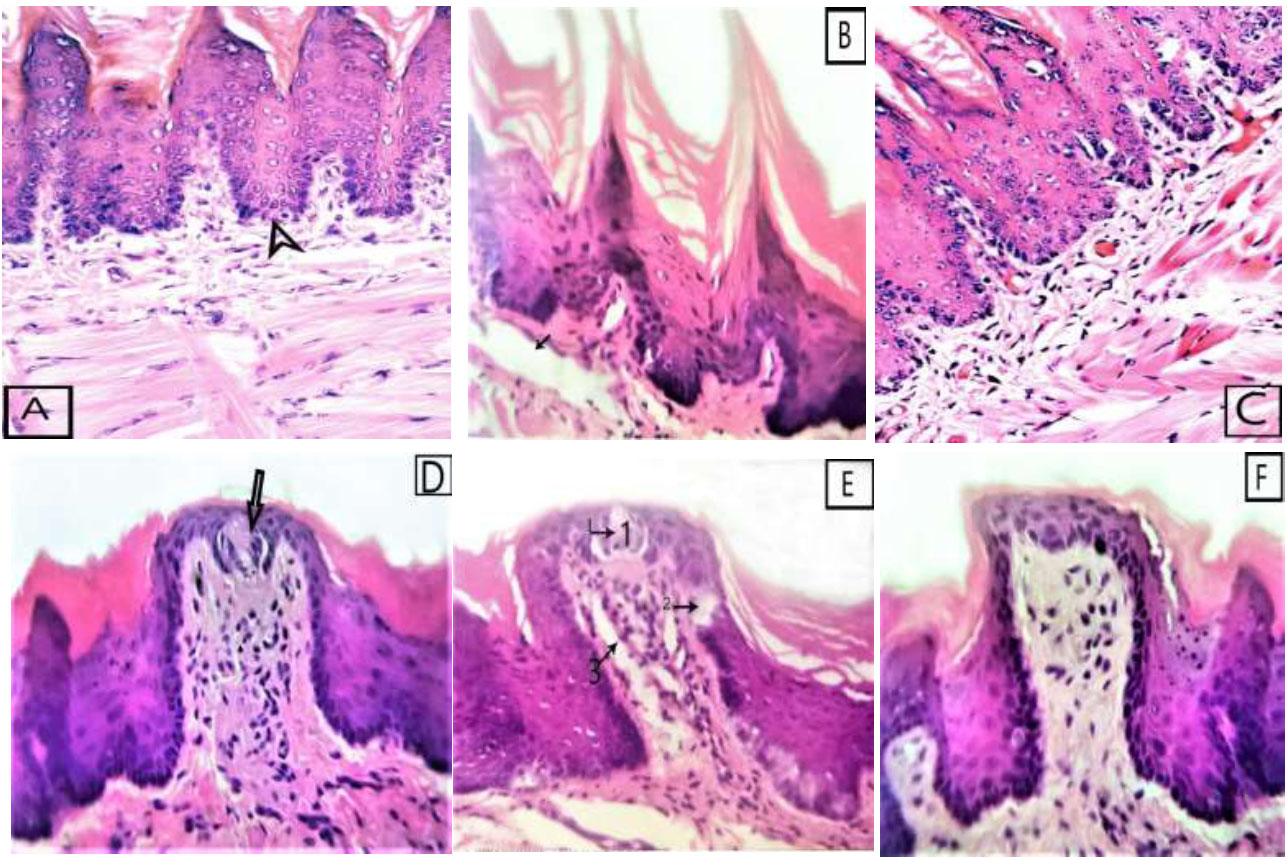
Photomicrographs of tongue dorsal surface, (A&D) of group I showing (A) full thickness of epithelium with regular cover of conical filiform and normal keratin thickness, deep rete pegs (arrowhead) (D) and fungiform papilla with one taste bud in its superior surface (arrow), (B&E) of group II showing (B) atrophied epithelium an irregular thin keratin layer and focal area of destruction in the connective tissue (arrow), (E) fungiform papillae with destructed taste bud cells (arrow 1),destructed basal cells (arrow 2) and destructed connective tissue (arrow 3), (C&F) of group III showing (C) full thickness of epithelium with regular cover of filiform papilla and normal connective tissue and muscle fibers, (F) showing regeneration of normal architecture of fungiform papillae with normal rete pegs (H&E x400).
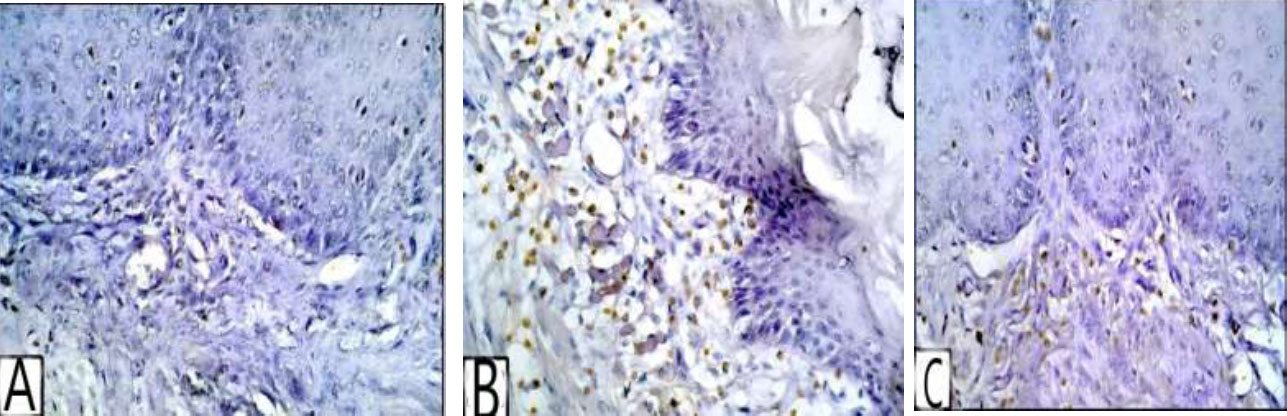
(A) Photomicrograph of group I showing no expression of CD3 in the laminae propria, (B) showing intense CD3 expression in the laminae propria of group II, (C) showing group III with moderate expression of CD3 in the laminae propria (CD3 reaction for T lymphocyte x400).
| - | Control | Methotrexate | Flax Seeds |
|---|---|---|---|
| Area % | 0.53±0.31*** | 1.78±0.55***### | 1.38±0.62 |
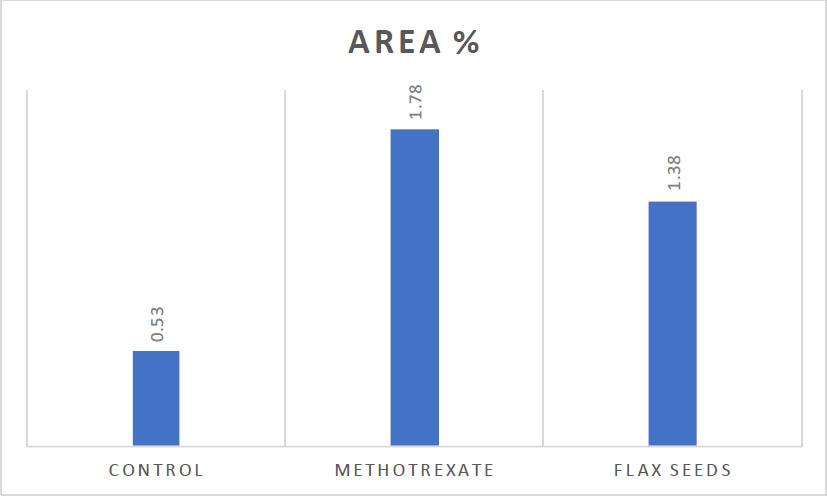
Clustered column showing the statistical analysis for CD3 immunostaining denotes statistical significance.
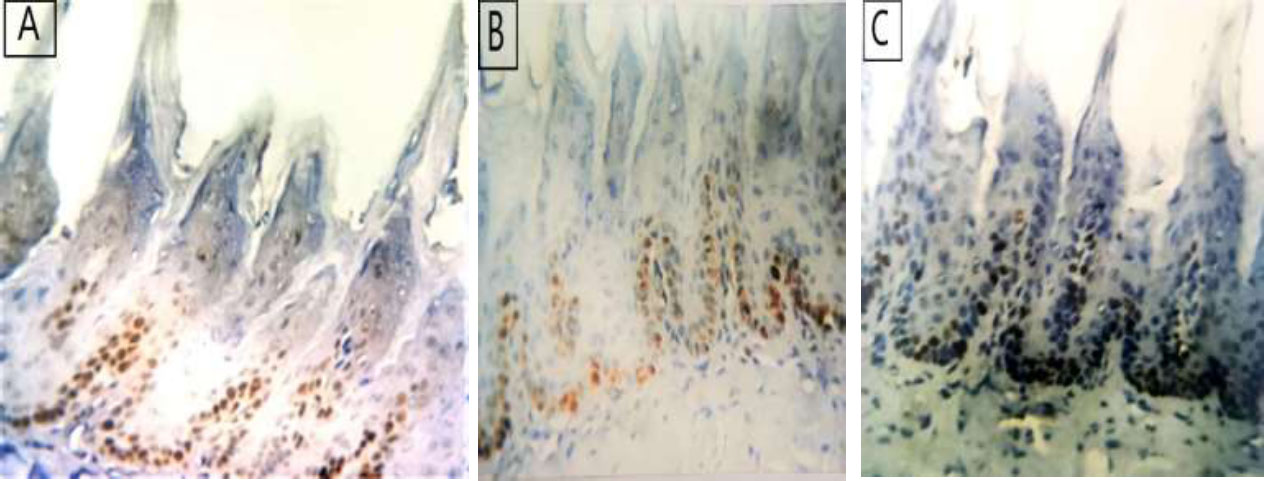
(A) photomicrograph of group I showing moderate expression of PCNA in the basal cell layer and parabasal cell layer, (B) showing weak expression of PCNA in basal cell layer of group II, (C) showing group III with intense expression of PCNA in the basal and parabasal cell layer (PCNA reaction x400).
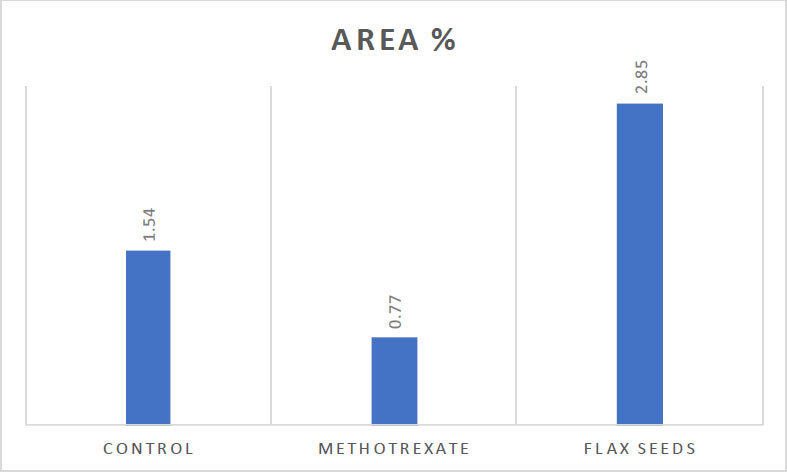
Clustered column showing the statistical analysis for PCNA immunostaining Denotes statistical significance.
| - | Control | Methotrexate | Flax Seeds |
|---|---|---|---|
| Area % | 1.54±0.24 | 0.77±0.64*** | 2.85±0.43***### |
Flaxseed and its derivatives, flaxseed oil and seed meal, are rich sources of the essential fatty acid, Alpha-Linolenic Acid (ALA), a natural precursor to omega-3 fatty acids such as eicosapentaenoic acid (EPA). Various animal studies have shown that omega-3 fatty acids derived from flaxseed exhibit anti-atherosclerotic, renoprotective, anti-inflammatory, and immunomodulatory effects, offering protection against conditions such as Crohn's disease, ulcerative colitis, and rheumatoid arthritis. Flaxseed also contains lignans, particularly secoisolariciresinol diglucoside (SDG), a carbohydrate-based polyphenolic compound. SDG is a potent free radical scavenger and has been shown to enhance the body’s defense mechanisms, providing protective effects against various diseases, including certain types of cancer [27].
The current investigation aimed to explore the possible preservative effects of flaxseed on methotrexate-induced inflammation in the tongues of albino rats.
The research results revealed that methotrexate induced atrophic and inflammatory alternations on the dorsal surface of the tongue. H&E-stained sections demonstrated a reduction in epithelial thickness, mild disintegration of the fungiform papillae with damaged taste buds, thin, detached keratin, and localized areas of destruction in connective tissue. These results align with those of a study by Hanaa Salem Moubarak, whose research proved that methotrexate causes degenerative and inflammatory changes in both the buccal mucosa and the anterior region of the tongue dorsum [19].
PCNA is a 36-KDa protein known as cyclin. It is a distinctive marker for cellular expansion and is found in nearly all proliferation cell phases. This protein is integrated in the early G1 to S phase of the cell cycle [28]. PCNA is the most favorable marker of cell proliferation in normal and neoplastic tissues associated with the cell cycle [29].
CD3 antibody is a complex consisting of at least 5 transmembrane protein polypeptides associated with T-lymphocytes and linked to the T-cell receptor. This CD3 complex encompasses the γ, δ, ε, ζ, and η chains (subunits). When an antigen binds to the T-cell receptor, the CD3 complex facilitates signal transduction to the cytoplasm of the T-cell [30].
Immunohistochemical analysis revealed that MTX induced a noteworthy increase in CD3 and a remarkable decrease in PCNA immunostaining in comparison with a control group. These results were in accordance with the results of a study by Ahmed et al., who found that MTX-treated rats revealed weakly positive PCNA staining in the nuclei of the basal and parabasal cells of the surface epithelium [31].
Moreover, the results of CD3 in this study were also corroborated by Sachiko Furukawa et al., who reported a case involving prolonged Methotrexate (MTX) treatment (4 years). The immunohistochemical analysis of the case revealed considerable lymphocytic infiltration, with T cells identified as CD3-positive [32].
Notwithstanding, in the treated group of the current study, flaxseeds attenuated the atrophic and inflammatory alterations caused by MTX, as demonstrated by the histopathological and immunohistochemical staining results. H&E sections revealed a restoration of the normal morphology and thickness of lingual mucosal tissues. Moreover, immunohistochemical analysis demonstrated notable improvement in PCNA immunoreactivity, along with a significant reduction in CD3 levels.
Kuduban et al. explained these findings by noting that during the administration of MTX, key cytokines levels in the blood increase, leading to tissue damage [33]. Moreover, Vu TH and Werb Z reported that MMPs alter the microenvironment and affect cellular behavior by interacting with ECM molecules, influencing cell shape and proliferation. This might explain the reduced PCNA response in group II compared to groups I and III [34].
The anti-inflammatory effects of flaxseeds evidenced in our study align with the findings of Mihir Parikh et al., who asserted that flaxseed is rich in omega-3 fatty acid, alpha-linolenic acid, the lignan secoisolariciresinol diglucoside, and fiber. These compounds contribute to the significant biological activity that benefits the health of both animals and humans through their anti-inflammatory action, anti-oxidative properties, and lipid-regulating effects [35]. Furthermore, Purushothaman et al. reported that the α-linolenic acid in flaxseed is broadly used as an anti-inflammatory agent by reducing the production of inflammatory cytokines, lipids, and lipoprotein [36].
The increased cellular proliferation observed in Group III (MTX/Flaxseed), compared to Group II (MTX-treated rats), aligns with the findings of Radoslava Vlčková et al., who reported that flaxseed promotes PCNA expression in all components of secondary follicles and in the corpora lutea of ovaries in mice fed with flaxseed [37].
Moreover, the findings of this study corroborate those of Hamza Mechchate et al., who demonstrated that the free polyphenol fraction of flaxseeds (PLU) exerts an anti-inflammatory effect during both the first and second phases of the carrageenan-induced inflammatory response following carrageenan injection into the hind paw of rats [38].
Considering the previously discussed results, our study demonstrated that flaxseeds exert anti-inflammatory and anti-mucositis effects on the lingual mucosa of MTX-treated rats. These findings are supported by M. Mohameda et al., who showed the anti-inflammatory potential of flaxseed oil compared to two other vegetable oils in a rat model of indomethacin-induced gastric ulceration. In the indomethacin-treated group, several histopathological abnormalities were observed, including significant infiltration of mononuclear inflammatory cells in the mucosa, mucosal necrosis, hemorrhage, and widespread submucosal edema. In contrast, the flaxseed-treated groups exhibited a marked reversal of these damages, with the restoration of normal stomach tissue histology. Sections from these groups showed improvement in the lesions, with only mild mucosal and submucosal inflammatory cell infiltration and edema [39].
CONCLUSION
The administration of flaxseed could potentially prevent or reduce MTX-induced mucositis toxicity. The results of this study provide experimental evidence that flaxseeds reduce inflammation and oxidative stress, as well as improve the histopathological alterations caused by MTX. These antioxidant and anti-inflammatory effects may be attributed to the presence of phenolic compounds and unsaturated fatty acids in flaxseeds.
LIMITATIONS OF THE STUDY
This research was conducted using an animal model, which, while providing valuable insights, limits the direct applicability of the findings to humans. Furthermore, despite numerous studies on the nutritional composition and health benefits of flaxseed, there remains a lack of comprehensive knowledge regarding its biologically active constituents, their safety profiles, environmentally friendly extraction methods, and the range of diseases they can potentially manage. Additionally, the development of functional food products incorporating flaxseed in various forms is still underexplored. Therefore, further studies are needed to investigate the pharmacokinetic and toxicological properties of flaxseed and its efficacy against MTX-induced mucositis.
AUTHORS’ CONTRIBUTIONS
The authors confirm their contribution to the paper as follows: S.H.A.B.: Study conception and design:; A.A.R.M.: Analysis and interpretation of results. All authors reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| MTX | = Methotrexate |
| GFR | = Glomerular filtration rate |
| IL-1 | = Interleukin 1 |
| TNF | = Tumor necrosis factor |
| ALA | = α-linolenic acid |
| EPA | = Eicosapentaenoic acid |
| DHA | = Docosahexaenoic acid |
| LA | = Linoleic acid |
| AA | = Arachidonic acid |
| GLA | = Gamma-linolenic acid |
| SDG | = Secoisolariciresinol diglucoside |
| PCNA | = Proliferating cell nuclear antigen |
| ECM | = Extracellular matrix |
| EPA | = Eicosapentaenoic Acid |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All research steps were conducted in accordance with the Mansoura University Animal Care and Use Committee, Egypt (Approval No. MU-ACUC (DENT.R.24.12.18).
HUMAN AND ANIMAL RIGHTS
This study adheres to internationally accepted standards for animal research, following the 3Rs principle. The ARRIVE guidelines were employed for reporting experiments involving live animals, promoting ethical research practices.
The animal experimentation was conducted according to the Guide for the Care and Use of Laboratory Animals.
AVAILABILITY OF DATA AND MATERIALS
The datasets generated and/or analyzed during the current study are available from the corresponding author [A.M] upon reasonable request.
ACKNOWLEDGEMENTS
Declared none.


