All published articles of this journal are available on ScienceDirect.
Effects of High-concentration Doxycycline versus Chlorhexidine as Dentin Pretreatment on Composite Resin Micro-tensile Bond Strength
Abstract
Objective
To assess the effect of 10% doxycycline (DOX) compared to 2% chlorhexidine (CHX) on the longevity of dentin-bond strength.
Methods
Sixty-six extracted molars were collected and prepared and divided into two groups based on the test to be performed. Group I (n=48) for the micro-tensile bond strength (µTBS) test and Group II (n=18) for the confocal laser scanning microscopy (CLSM) test. Group I was divided into 3 subgroups (n=16) according to pretreatment agent following acid etching. Group A (control): without pretreatment, group B: 10% Doxycycline pretreatment, Group C: 2% Chlorhexidine pretreatment. Palfique universal bond (PU) and Composite resin were applied according to the manufacturer's instructions. Group II was divided into 3 subgroups (n=6) based on the same pretreatment protocols used in Group I. However, for resin-dentin bond evaluation using CLSM, 0.1 mg/mL Rhodamine B fluorescent dye was mixed with the adhesive. After that, all subgroups of group I and group II were subdivided into 2 subdivisions:(T1) without thermocycling and (T2) thermocycling for 10000 cycles. µTBS testing was achieved via a digital universal testing machine. The failure mode was tested by a stereomicroscope (30x magnification).
Results
Intergroup comparison of mean µTBS values (MPa) was performed using one-way ANOVA, then by Tukey post-hoc test with statistical significance at p ≤ 0.016. In contrast, intragroup comparison was achieved using a paired t-test with a statistical significance of p ≤ 0.05. Comparison between categorical data was made using a chi-square test. The 10% DOX pretreatment group showed significantly higher µTBS values than CHX and the control group with and without thermocycling (p < 0.001). No statistically significant difference in failure modes was recorded among groups without and with thermocycling. There was no correlation between µTBS and failure mode performed using Spearman’s rank correlation. CLSM revealed that the 10% DOX group exhibited greater resin infiltration with a thicker hybrid layer. Both matrix metalloproteinase (MMP) inhibitors created a uniform hybrid layer.
Discussion
10% DOX showed the highest MMP inhibition, the higher concentration of DOX may have increased the inactivation effect of DOX on MMPs.
Conclusion
Pre-treatment with MMP inhibitors might have inhibited the degradation of resin-dentin interfaces. In addition, 10% DOX pretreatment for 60 seconds after etching appears to be more efficient in enhancing the durability of the bond. Additionally, the composition of PU may influence bond strength, warranting further investigation. Moreover, thermocycling may adversely impact the micro-tensile bond strength.
1. INTRODUCTION
The advent of adhesive systems has ushered a paradigm shift in conservative dentistry, offering an array of revolutionary capabilities that were previously beyond imagination. These cutting-edge solutions permit clinicians to bond with tooth structure without relying on retentive cavities, bringing in an era of minimally invasive dental procedures [1]. Hence, the retention of adhesive restorations is determined by the bond strength between the tooth structure and the restorative material. In most clinical procedures of cavity preparation, dentin is the principal substrate. Therefore, ensuring the strength and stability of both the hybrid layer's dentin and adhesive components will forever be crucial for obtaining the bond’s long-term perseverance [2]. The dentin-resin bond is considered a tissue remodeling method wherein demineralized dentin is used as a scaffold to allow resin infiltration. The idea is to create a hybrid layer that will hold resin composites or adhesives firmly to the deeply mineralized dentin [3].
A combination of mechanical and chemical forces causes the hybrid layer to degrade, including procedures like the enzymatic and hydrolytic disintegration of the uncovered dentin collagen fibrils [4]. This degradation may begin immediately after bonding or even following adhesive polymerization [5]. In the first method, acid etching leads to the gradual breakage of collagen fibrils by MMPs, which are activated by this process [6]. Conversely, the later stages of degradation are initiated by the hydrolysis of vulnerable groups such as carboxyl, hydroxyl, and phosphate functionalities within the molecular structure of methacrylate-based resin monomers [7].
Enzymes are essential for the hybrid layer's dissolution, particularly MMPs, which are metal-dependent proteolytic enzymes that can break down collagen. They can be divided into six subgroups according to their substrate specificity. Within these categories are collagenases (MMP-1 and MMP-8) and gelatinases (MMP-2 and MMP-9) [8]. Among these, MMP-2 is extremely prevalent, and MMP-9 [9]. Consequently, many studies imply that suppressing MMP activity may improve the hybrid layer's stability.
Many proposals aim to improve bond stability, leading to the clinical success of resin restorations [10]. Accordingly, the CHX mode of action entails inhibition of enzyme activity on dentin matrices, thus improving the hybrid layer's longevity and bond stability [11]
DOX is classified as an antibiotic within the tetracycline family and is recognized for its antimicrobial potency and broad-spectrum inhibition of MMPs. The strategy involving dentin pretreatment with DOX characterized by the pathological involvement of MMPs was proven [12, 13]. Furthermore, several studies have employed doxycycline as both an MMP inhibitor and to retard the degradation process at restoration interfaces (2). Based on research, it appears that DOX wouldn't weaken dentin's original bond [14]. Yet, the influence of the DOX effect on the stability of hybrid layer longevity has not been fully explored.
Therefore, this research goals were to assess the MMPs inhibition ability of two different dentin pre-treatments and to gain insight into the μTBS after thermocycling. So, the study objectives were to test the effect of a high percentage (10%) DOX solution compared to 2% CHX on the dentin μTBS before and after thermocycling. The examined first null hypothesis would be that DOX pretreatment poses a similar effect on dentin bond strength as CHX. The second null hypothesis would be that thermocycling does not affect dentin bond strength.
2. MATERIALS AND METHODS
The research ethics board of King Abdulaziz University's College of Dentistry in Jeddah, Saudi Arabia, approved this study under ethical number 45-98946.
2.1. Tested Chemicals
10% DOX solution preparation: 10% (w/v) of DOX (Sigma, St. Louis, MO, USA), approved by the FDA [15]. It was allowed to dissolve for two hours at 50°C while stirring in phosphate-buffered saline (PUS; pH 7.2, Pittsburgh, PA, USA) solution [16].
Consepsis: 2% CHX gluconate antibacterial solution (Ultradent Products, USA).
2.2. Sample Size
In a previous study by Sharifian et al. (2023), the micro-tensile bond strength (µTBS) in the etch-and-rinse group showed a normal distribution, with a standard deviation of 1.83 [17]. Assuming a mean difference of 3 between the experimental and control groups, and setting the statistical power at 0.80 to detect this difference, a minimum of seven samples per subgroup was calculated. To account for the possibility of a non-parametric distribution, the sample size was raised by 15%, reaching 8 samples per subgroup. Type I error probability would be 0.05 to test the null hypothesis. Independent t-test, PS Power, and Sample for Windows version 3.1.6 were used to determine the sample size.
2.3. Specimens’ Preparation
Sixty-six recently extracted, human molars free of caries were stored in a 0.1% thymol solution. A model trimmer (Model Trimmer, Sejong, Korea) was used to flatten the occlusal enamel and remove the occlusal third underwater flow to obtain a flat exposed dentin surface. For smear layer standardization, 600-grit silicon carbide abrasives were used [18]. After that, teeth were placed into a mold made of self-curing acrylic resin. As for μTBS testing, forty-eight teeth were used and randomly distributed based on the dentin pretreatment into 3 groups n=16. Group A: no pretreatment (control), Group B: 10% DOX pretreatment, and Group C: 2% CHX pretreatment. In compliance with the manufacturer's recommendations, dentin surfaces were etched using 37% phosphoric acid before bonding (Table 1). Control group A (n = 16) received no pretreatment. Group B: DOX (n = 16): 10% DOX solution was brushed onto the dentin surface by a micro-brush for 60 seconds. A continuous, 2-second moderate airflow was used to dry the dentin surface. Group C: CHX (n = 16), by micro brush, 2% CHX was added to the exposed dentin for 60 seconds, then left to air dry for 2 seconds [20]. PU was applied per the manufacturer's standards (Table 1).
Dental composite Palfique LX5, Supra-nano composite (Tokuyama Dental Corporation, Taitō-Ku, Tokyo, Japan) was used to build composite blocks above the dentin in 2 mm for 2 increments. Light curing for 20 seconds to each increment using light intensity of 1,000 mW/cm2 using a light-emitting diode (LED) curing device (Mini LED, Satelec, Acteon, France) was done. A radiometer X (SDI Limited, Australia) was used to caliper the light curing unit after every five specimens. Then, each group was divided into 2 subgroups, where a group of specimens (n=8) was immersed at 37°C for 24 hours in distilled water, while the other group underwent 10,000 cycles of thermocycling [19]. The temperature varied with dwell periods of 15 seconds and intervals of 10 seconds, ranging from 5 to 55 degrees Celsius (Fig. 1).
2.4. Micro-tensile Testing
Restored teeth were secured with a specially constructed grasping attachment parallel to the sectioning direction, preserving the perpendicular relationship between the cutting disc and the occlusal surface. Metal house with two screws for secure attachment of acrylic blocks soldered at a square base to ensure standard cutting in bucco-lingual and mesio-distal directions at 90 degrees to each other. A diamond-coated disc (Isomet 4000, Buehler, Germany) of 0.3 mm was utilized, serially sectioning the beams to 0.8 mm thickness. A digital caliper (Total Tools, Malaysia) was used to ensure all beam dimensions. Each beam was preserved in distilled water inside a tightly sealed labeled tube. Then, a jig was used to bond each beam from the ends with cyanoacrylate-based glue (Akfix 705 quick adhesive, Turkey) at least 1 mm from the adhesive interface. The glue was allowed to dry more quickly by using a glue accelerator. The jig was placed in the universal testing machine (Instron, model 3345, MA, USA), which had a 500 N load cell until failure. A tensile load was applied at a cross-head speed of 0.5 mm/min. Bond strength results were recorded using MegaPascal (Bluehill Lite program, Instron, MA, USA).
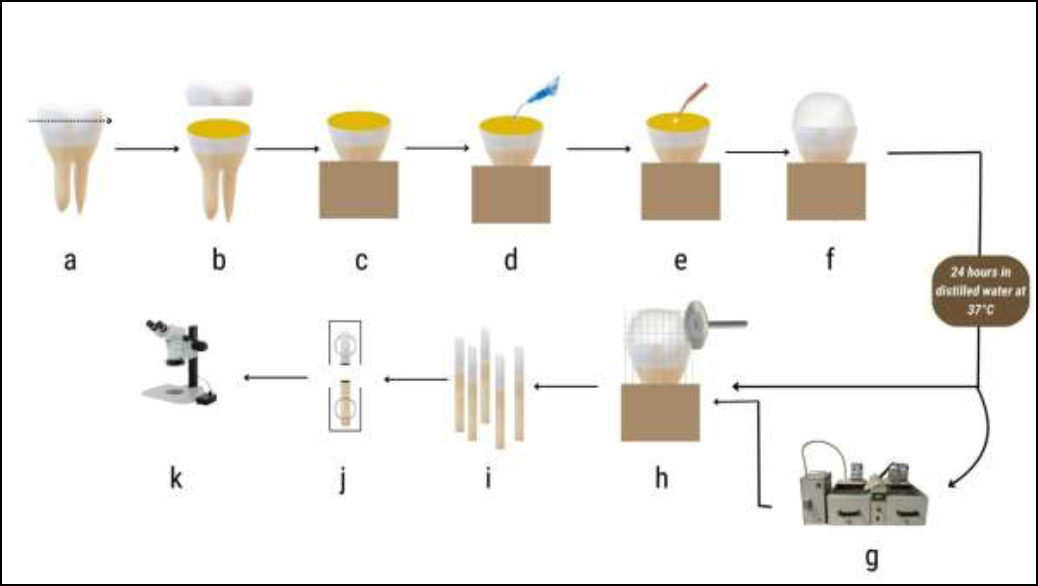
Flowchart for the μTBS test specimen preparation. (a) tooth; (b) trimming of occlusal third; (c) exposed dentin; (d) etching of dentin; (e) dentin pretreatment application &/or bond application; (f) composite application; (g) thermocycling; (h) tooth sectioning; (i) beams 0.8 mm thickness; (j) beam after μTBS testing (k) failure mode observation.
| Material | Composition | Manufacturer | Application Strategy |
|---|---|---|---|
| Palfique LX5 | 82% by weight Supra-nano spherical filler, monmer: Bis-GMA, Triethylene glycol dimethacrylate | Tokuyama Dental, Tokyo, Japan (Lot no. 022E61) |
1. Placed in increments of 1mm 2. Each increment cured for 20 s. |
| Palfique Bond (PB) |
Bottle 1 (Bond):3D-SR, MTU-6, HEMA, BIS-GMA, TEGDMA, Water. Bottle 2: Y-MPTES, Borate, Peroxide, Acetone, Isopropyl alcohol added to Water. |
Tokuyama Dental, Tokyo, Japan | 1. Adhesive from two bottles (Bottles A and B). Dispense a single drop per bottle, mix, and rub for 5 seconds.2. Dry with air the adhesive for five seconds to let the solvent dissipate. |
| Scotchbond Etchant | 37% phosphoric acid | 3M ESPE | 1. Apply acid for 15 seconds; 2. Rinse for 10 seconds; 3. Use an air jet to remove any remaining water for 2 seconds; |
| CHX (Consepsis solution) | 2% Chlorhexidine gluconate solution | Ultradent Products Inc., USA | 1. Use a microbrush to apply to the dentin surface for 60 seconds. 2. Give it a two-second air dry. |
| DOX | 10% DOX solution | Sigma, St. Louis, MO, USA | 1. Apply to dentin surface with a micro-brush for 60s. 2.Gently air dry for 2 s. |
2.5. Failure Mode
The fractured beams were inspected by MA 100 Nikon stereomicroscope under magnification of 30X and categorized as either:
A) Mixed failure between adhesive and restorative material
B) Dentin cohesive failure
C) Cohesive failure of restorative material
D) Adhesive failure at the restoration interface
2.6. Confocal Laser Scanning Microscopy (CLSM) testing
Eighteen teeth were divided at random into 3 groups, n=6. Specimens were prepared the same as the previously described method for micro-tensile specimens’ preparation, except sectioning them in buccolingual only. Also, the adhesive was mixed with approximately 0.1 mg/mL of Rhodamine B fluorescent dye (Loba Chemie PVL. Ltd., Mumbai, India) before application. Then, the adhesive layer was thinned for 5 seconds with an air stream, and composite resin was applied. Half of the teeth from each group n=3 were thermo-cycled for 10,000 cycles [20]. All specimens have been investigated using confocal microscopy (Leica Microsystems CMS GmbH, Mannheim, Germany) at excitation of 540 nm and emission of 590 nm wavelengths.
2.7. Statistical Analysis
For data analysis, version 22 for Windows Medcalc software (MedCalc Software Ltd, Ostend, Belgium) was utilized. For data normality, Shapiro-Wilk and Kolmogorov-Smirnov tests were done. The mean and standard deviation were used to characterize continuous data with normal distribution. For intergroup comparison of constant data, one-way ANOVA was done. After Bonferroni correction, the Tukey post-hoc test was completed with statistical significance set at p ≤ 0.016 for the intragroup comparison. A paired t-test with statistical significance at p < 0.05 was cast for the intragroup comparison. Both percentage and frequency were used to characterize data. A chi-square test with statistical significance set at p < 0.016 after Bonferroni correction was used for an intragroup comparison of categorical data, and a chi-square test with statistical significance set at Pat p ≤ 0.05. Using Spearman's rank correlation, the correlation between bond strength values and failure mode was examined [21].
3. RESULTS
3.1. Micro-tensile Bond Strength
Table 2 and Fig. (2) present the mean values of μTBS. 10% DOX pretreatment showed a higher statistically significant µTBS (32.80 MPa) compared to CHX (24.70 MPa) and control group (20.96 MPa) without thermocycling at (p < 0.001). While with thermocycling, 10% DOX showed higher statistically significant µTBS (24.05MPa), compared to CHX (15.49 MPa) and the control group (12.67 MPa) at (p < 0.001). Intragroup comparison within each group has shown a significant difference statistically between those without thermocycling and those with thermocycling (p < 0.0001, p = 0.0001, and p < 0.0001) correspondingly.
3.2. Failure Mode
Failure modes analysis was done using stereomicroscope evaluation. Table 3 and Figs. (3 & 4) show the percentage of failure modes of different groups. Intergroup comparisons between the control and dentin pretreatment groups revealed no statistically significant differences in failure modes with or without thermocycling (p = 0.0907 and p = 0.2483). Intragroup comparisons within each group have shown no statistically significant difference between without thermocycling and with thermocycling (p = 0.4707, p = 0.1026 and p = 0.4322) correspondingly.
Finally, for correlation among μTBS and failure mode performed using Spearman’s rank correlation or Spearman’s rho revealed negligible or no correlation (rho=0.166, p= 0.3330).
3.3. Confocal Microscopy Results
Resin dentin interface was observed, and random specimens from each group were tested (Fig. 5). Specimens with and without thermocycling exhibited hybrid layer and resin tag formation. DOX group showed longer and thicker resin tags hybrid layer in comparison with the CHX and control group. However, specimens subjected to thermocycling showed fewer resin tags and weaker bonds, indicating that thermocycling boosted the degree of enzymatic activity.
| ABA Aging |
Control | DOX | CHX | p-value | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | - | |
| T1 | 20.96c | 1.10 | 32.80a | 0.93 | 24.70b | 0.83 | < 0.001* |
| T2 | 12.67c | 1.51 | 24.05a | 1.54 | 15.49b | 0.49 | < 0.001* |
| p-value | < 0.0001* | = 0.0001* | < 0.0001* | - | |||
| ABA Aging |
Control | DOX | CHX | p-value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AD | CC | CD | M | AD | CC | CD | M | AD | CC | CD | M | - | |
| T1 | 2(12.5%) | 2(25%) | 4(25%) | 8(50%) | 0(0%) | 8(50%) | 3(18.8%) | 5(31.2%) | 4(25%) | 7(43.8%) | 1(6.2%) | 4(25%) | =0.0907 |
| T2 | 4(25%) | 2(12.6%) | 1(6.2%) | 9(56.2%) | 5(31.2%) | 5(31.2%) | 3(18.8%) | 3(18.8%) | 5(31.2%) | 4(25%) | 0(0%) | 7(43.8%) | =0.2483 |
| p-value | = 0.4707 | = 0.1026 | = 0.4322 | - | |||||||||
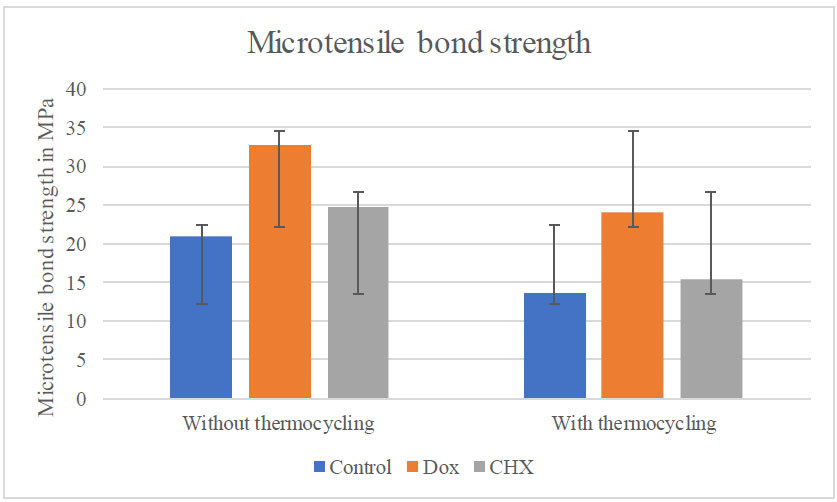
Bar chart showing μTBS and SD of each dentin pretreatment within each aging group.
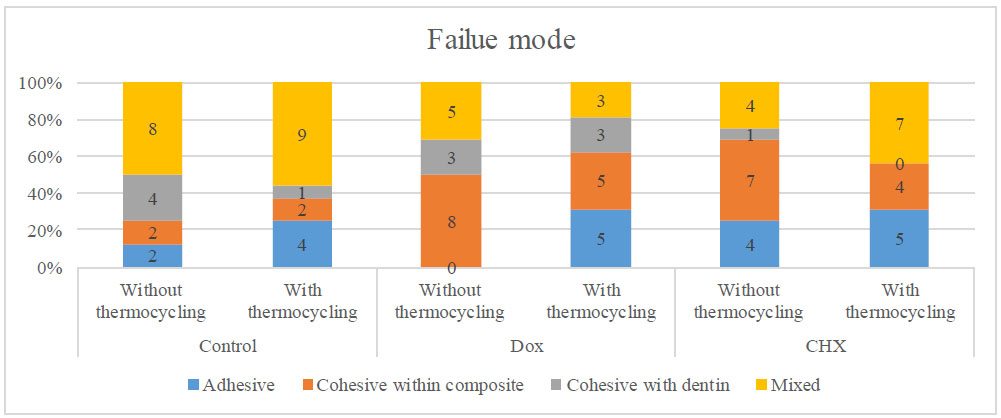
Bar chart showing the distribution of failure mode of each dentin pretreatment within each aging group.
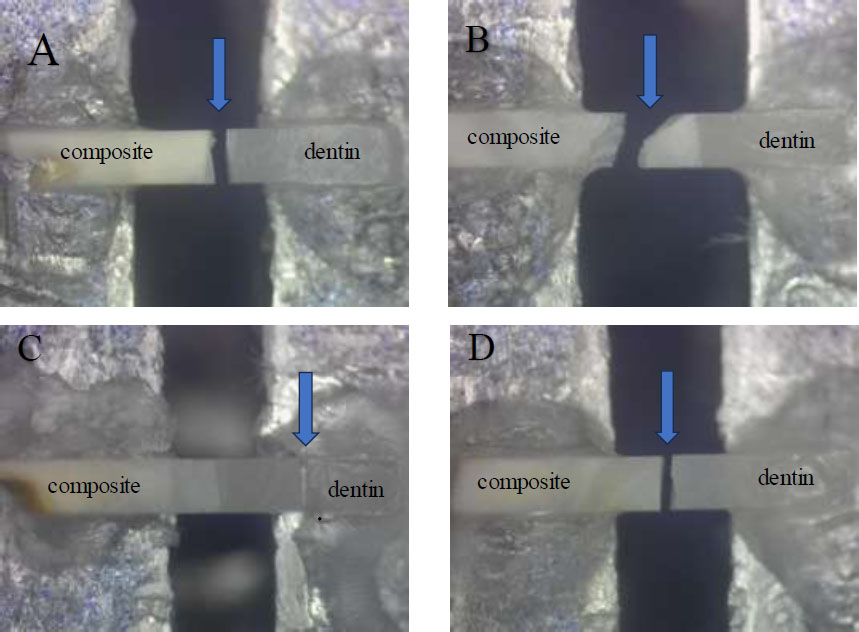
Showing different fracture modes (Stereomicroscope images). A: mixed fracture, B: Cohesive fracture within the composite, C: Cohesive fracture within the dentin, and D: adhesive fracture.
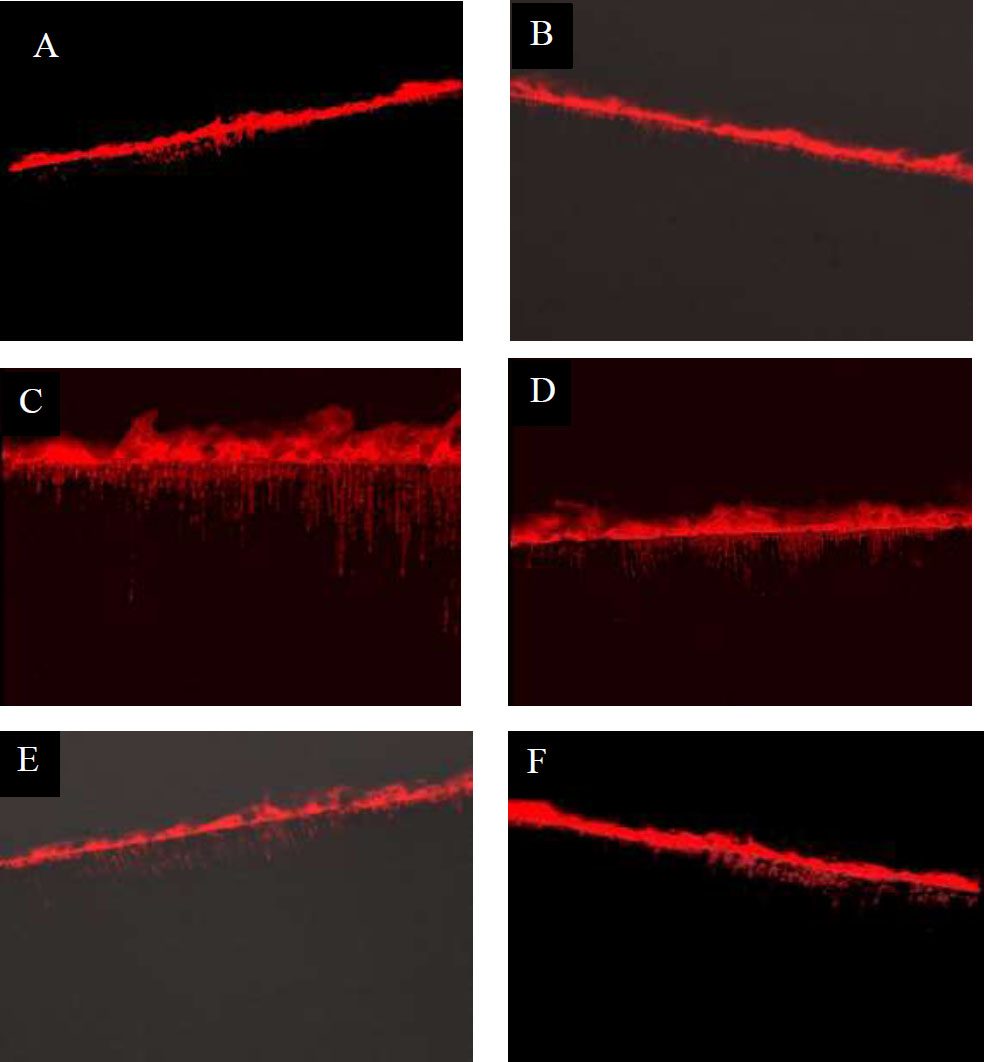
Showing resin-dentin interfaces before thermocycling (T1) and after thermocycling (T2) (Confocal laser scanning microscopy images). A: Control (T1), B: Control (T2), C: Dox (T1), D: Dox (T2), E: CHX (T1) and F: CHX (T2).
4. DISCUSSION
The hybrid layer’s biodegradation poses a challenge to maintaining the longevity of dentin bonding. In the current study, the effect of two dentin pretreatments, known as MMPs inhibitors, on µTBS of dentin-resin was measured after 24 hours and then after thermocycling. Despite the recent emergence of simpler adhesive solutions, total-etch and rinse adhesives are still considered the golden standard regarding endurance and linking strength. During bonding, the collagen fibrils in the organic matrix are revealed by the acidic monomers [22]. For the hybrid layer formation, the adhesive resin monomer should infiltrate into the exposed collagen network. However, inadequate resin infiltration may persist at the hybrid layer. The resulting denuded collagen fibrils are susceptible to degradation by host-derived matrix metalloproteinases (MMPs), which are activated by phosphoric acid or acidic primers, leading to dentin demineralization [23].
During the bonding procedure, phosphoric acid/acidic primers demineralize dentin and activate MMPs, which break down denuded type I collagen. The bonded dentin matrix in the hybrid layer is degraded by the actions of endogenous collagenase and gelatinase produced from demineralized dentin [24]. As a result, the hybrid layer loses its solidity over time, causing failure of the resin composite restoration. Therefore, the use of specific MMP inhibitors is recommended to prevent degradation of the hybrid layer by suppressing collagenolytic and gelatinolytic activity in dentin.
In the present study, the control group had the lowest µTBS, which could be due to the functional monomer of the adhesive that has a direct impact on the effectiveness of the adhesive bond. The functional monomer 3D-SR of the PU is based on three-dimensional (3D) self-reinforcing (SR) technology that could, in part, self-arrange within the adhesive to form monomeric structures with various phosphate groups that can interact with calcium at multiple places to form ionic bonds. However, this result aligns with findings by Santander-Rengifo et al. and Campos et al., who reported that adhesives containing the 10-MDP functional monomer demonstrated superior bond strength compared to those based on 3D-SR monomers [25, 26].
In addition, the evaporation of isopropyl alcohol takes longer than that of ethanol due to its lower vapor pressure. The solvent may not have been eliminated by the air jet, which could have resulted in the formation of pores in the polymerized adhesive nanolayer. These pores would have decreased the bond strength of the adhesive layer [27, 28].
The deceased bond strength of PU, yet not fully tested, may have been counteracted with dentin pretreatment with DOX and CHX that showed statistically significantly higher µTBS, respectively, than the control group.
The decreased bond strength of PU, although not fully explored, may have been compensated for by dentin pretreatment with DOX and CHX, both of which showed statistically significantly higher µTBS compared to the control group.
DOX, a semisynthetic tetracycline approved by the FDA for the treatment of periodontal disease, can suppress collagenase enzymes such as MMPs [29]. In this study, 10% DOX demonstrated the highest level of MMP inhibition. The higher concentration of DOX may have increased the inactivation effect of DOX on MMPs [30]. Moreover, DOX can bind with enzyme-associated calcium and zinc and inhibit the proteolytic action of MMPs forms [29, 30]. DOX reacting with Ca and Zn has a potent, long-lasting inhibition effect compared to CHX, which reacts with the catalytic Zn. Therefore, DOX impedes the endogenous proteases, thus preserving collagen from degradation and improving bond strength [29]. The results followed Loguercio et al. and EL-Behairy et al., who reported that using 2% minocycline and 2% CHX as dentin pretreatment can slow the resin-dentin interface degradation, contradicting some research that claimed DOX negatively impaired the bonding interface [31, 32]. Stanislawczuk et al. stated that low bond strength values resulted after mixing DOX with bonding agents due to phase separation [33]. El Zohairy et al. concluded that the resin monomer infiltration was not compatible with the increased depth of demineralization [34]. Furthermore, a limitation of Dox is that it can cause a noticeable brownish discoloration at the adhesive interface, which may affect the clinical aesthetics of the restoration [2].
On the other hand, the µTBS values of the CHX-treated groups were significantly higher than those of the untreated groups, both immediately and after thermocycling, but still statistically lower than those of the 10% DOX group. The effect of CHX on bond strength aligns with the findings of Breschi et al. and Ebrahimi et al. [35, 36]. Using CHX could maintain the bond interface, owing to the suppression of MMPs present in demineralized dentin [37, 38]. By binding to the collagen matrix negative carboxyl groups and the calcified dentin crystallites phosphate groups, CHX maintains a significant affinity for dental tissue and can inhibit dentin's gelatinolytic and collagenolytic activities, restricting the breakdown of hybrid layers [39]. Moreover, CHX's ability to saturate protease binding sites at any concentration and remain bound to collagen fibrils for delayed release at higher doses explains its long-term effectiveness [40]. In addition, CHX water-based solution is considered a rehydrating agent preserving the humidity required for the dentin to prevent the collapse of the collagen network and maintain it in an expanded condition [41]. This result was opposed to Giacomini et al. and Eltawary et al., who found that the application of 2% CHX had an adverse effect on bond strength [42, 43]. They found that the combined application of CHX and MDP-containing adhesives heightened collagenolytic and gelatinolytic activities, in addition to increasing the hydrolytic degradation of collagen fibrils. Also, CHX may have influenced the formation of collagen-protective MDP–Ca salts. This observation aligns with previous research that examined the impact of CHX solution on the bonding strength of adhesive systems with dentin [44-46]. These conflicting results from previous studies may be attributed to variations in CHX concentrations, types of bonding systems, storage media, and experimental methods.
Beyond that, thermocycling negatively affected all tested groups, so the second null hypothesis was rejected. The samples were subjected to a 10,000 thermocycles aging process between 5 and 55° C, representing acceptable standardized conditions that would simulate 6 months of clinical aging, predicting longevity and clinical success of restorative materials [47]. Thermocycling could stress the bond between the resin and dentin, accelerating water sorption and leaching of resin monomers, leading to a statistically significant decrease in µTBS compared to 24 h in all test groups [48]. However, µTBS values of DOX and CHX groups with thermocycling were statistically significantly higher than the control group, which may speculate their ability to inhibit the activity of MMPs, preventing collagen degradation over time. Moreover, HEMA presents the adhesive as hydrophilic and more liable for water degradation during water storage and thermocycling [49].
Regarding the resin dentin interface analysis with CLSM, the hybrid layer and adhesive resin penetration into dentin tubules were observed for all groups. The Dox group showed a continuous hybrid layer and greater diffusion of adhesive resins into dentinal tubules. However, pores were observed along the hybrid layer in nearly all groups, which may be attributed to the low vapor pressure of isopropyl alcohol in the PU adhesive, as previously mentioned [27, 28].
Observing the failure mode percentages revealed that all the test groups displayed almost identical failure values for all modes with intergroup and intra-group comparisons. This may verify that even when the cohesive force of the substrate material contributes to failure resistance, adhesive, cohesive, and mixed failures under load application are primarily caused by the separation of the resin-dentin beam at lower stress levels than those required to separate the substrate specimen [50]. This could be attributed to the low performance of PU, stated by many authors due to its chemical composition [25]
It was found that there was no correlation between µTBS values and failure modes. The various failure modes could be explained by the test mechanics and the material’s tendency to fracture rather than a sign of bond strength [51]. As for this study's limitations, for future studies, a larger sample size should be considered. It is recommended to compare self-etch with total-etch bonding and to employ extended aging with increased thermal cycles to validate the benefits of MMP inhibitors.
CONCLUSION
Under the limitations of this study, several conclusions can be drawn. Pre-treatment with MMP inhibitors such as DOX and CHX resulted in higher bond strength values in both 24-hour and aged specimens. Among them, a 10% concentration of DOX appeared to be more effective in enhancing the longevity of the dentin–resin bond. Additionally, the composition of PU may negatively influence bond strength, indicating the need for further investigation. Thermocycling was also found to have a significant detrimental effect on micro-tensile bond strength, highlighting the importance of considering aging protocols in evaluating adhesive performance.
CLINICAL IMPLICATIONS
While CHX improves dentin bond strength, 10% DOX may further enhance bond strength and durability. The degradation of the bonding interface is a significant clinical issue. Therefore, the promising results from MMP inhibitors should be clinically evaluated further. Extended follow-up periods are necessary to determine whether these products can be effectively integrated into restorative protocols.
AUTHOR’S CONTRIBUTIONS
The authors confirm contribution to the paper as follows: R. R. B. and D. M. A: Involved in the study’s conception, design, data collection, analysis, interpretation of results, and manuscript drafting; R. R. B.: Prepared the samples and testing procedure. Both authors reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| DOX | = Doxycyclin |
| CHX | = Chlorhexidine |
| μTBS | = Micro-tensile Bond Strength |
| CLSM | = Confocal Laser Scanning Microscopy |
| PU | = Palfique Universal Bond |
| MMP | = Matrix Metalloproteinase |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The research ethics board of King Abdulaziz University's College of Dentistry in Jeddah, Saudi Arabia, approved this study under ethical number 45-98946.
HUMAN AND ANIMAL RIGHTS
All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committees and with the 1975 Declaration of Helsinki, as revised in 2013.
AVAILABILITY OF DATA AND MATERIALS
The data that support the results of this study are accessible from the corresponding author [R.R.B] upon reasonable request.
ACKNOWLEDGEMENTS
Declared none.


