All published articles of this journal are available on ScienceDirect.
A Comprehensive Review of Biomaterials for Maxillary Sinus Floor Augmentation: Exploring Diverse Bone Graft Options
Abstract
Introduction
Improved histological analysis of bone substitutes has advanced maxillary sinus floor augmentation, leading to better clinical outcomes and implant stability. Careful material selection remains crucial for successful sinus lift procedures addressing posterior maxillary atrophy after tooth loss. This article provides a comprehensive review of the most commonly used materials for sinus lift procedures, focusing on their histological features and their impact on clinical results. The goal is to move beyond simply considering bone substitutes as replacement materials and to understand how their distinct properties enable the development of specific treatment approaches.
Methods
A literature review was conducted using electronic databases, such as PubMed/MEDLINE, Google Scholar, and the Cochrane Library (2018-August 2024). This review included English-language publications on maxillary sinus floor augmentation using various biomaterials. All clinical trials meeting the inclusion criteria were considered.
Results
This review included seven studies. The evaluated biomaterials demonstrated effectiveness and biocompatibility in maxillary sinus augmentation. Histological analyses revealed excellent osteoconductive properties, including the formation of new bone directly on the biomaterial surface and its integration into the existing bone structure, without evidence of inflammation.
Discussion
Each biomaterial category presents unique advantages and limitations. Autografts remain the gold standard but are constrained by availability. Allografts and xenografts provide viable alternatives, with xenografts favoring long-term stability and allografts balancing osteoconduction and osteoinduction. Alloplasts, particularly BCP and bioactive glasses, emerge as versatile options due to their tunable properties and biocompatibility. The findings underscore the importance of matching material properties to clinical needs, such as resorption rate and mechanical support.
Conclusion
A detailed understanding of the distinct properties of each graft material is crucial for selecting the most suitable bone substitute for maxillary sinus augmentation, resulting in improved clinical outcomes and a higher implant success rate.
1. INTRODUCTION
Insufficient bone dimensions in the edentulous posterior maxilla can hinder successful implant-supported rehabilitation.
Following tooth loss, the alveolar bone in the posterior maxilla undergoes resorption in both the horizontal dimension, due to remodeling of the buccal bone plate, and the vertical dimension. Vertical bone loss in the maxilla is often more pronounced than in other areas, leading to sinus enlargement at the expense of the alveolus. This occurs due to two factors, including increased osteoclastic activity in the periosteum of the Schneiderian membrane, and elevated positive intra-antral pressure during respiration resulting from tooth loss [1].
Therefore, bone grafting procedures are necessary to augment bone volume and offer the structural and mechanical support required for dental implant placement. The optimal bone graft should be biocompatible, osteoinductive, and osteoconductive. In addition, it should be fully replaced by new bone, able to maintain stable grafted volume, exhibit strong mechanical properties, originate from a patient-friendly source, and possess good handling characteristics. Among graft materials, autologous bone is regarded as the gold standard because of its osteogenic, osteoinductive, and osteoconductive properties [2].
However, autogenous bone grafting has notable limitations, including limited intraoral availability and donor site morbidity. Furthermore, it often requires general anesthesia for extraoral harvesting, involves longer operating times, necessitates two surgical sites, carries a risk of partial resorption, and poses potential intraoperative and postoperative complications [3].
To eliminate the need for a second surgical site for autogenous bone harvesting and reduce donor site morbidity, bone graft substitutes were introduced. These substitutes are categorized into three types: allografts, sourced from the same species (human) but a different individual; xenografts, derived from a different species, typically bovine; and alloplastic materials, which are of synthetic origin [4].
The optimal bone grafting material for maxillary sinus floor augmentation (MSFA) remains a topic of debate. Compromised bone quality and quantity in the posterior maxilla impede successful implant integration [5].
However, MSFA utilizing grafting materials promotes bone regeneration, thus increasing bone volume and enhancing the likelihood of achieving sufficient bone-to-implant contact (BIC) for long-term implant success [6].
Two primary methods are used to assess bone regeneration: histomorphometric analysis of tissue specimens and micro-computed tomography. Both techniques quantify the percentage of newly formed bone, non-mineralized tissue, residual grafting material, and bone-to-implant contact (BIC). Histomorphometric parameters are commonly reported as total bone volume (TBV) and bone area fraction (BAF), which is the proportion of newly formed bone, non-mineralized tissue, and residual graft material within a defined region of interest [7].
Experimental studies assessing histomorphometric variables and bone-implant contact following maxillary sinus floor augmentation with autogenous bone grafts compared to various other grafting materials have reported conflicting results, with none of the grafting materials demonstrating significantly superior histomorphometric characteristics [8-15].
The objective of this article is to present an overview of the most commonly used bone graft materials for maxillary sinus floor augmentation, based on biological, histological, and histomorphometric analyses.
2. METHODS
This review was carried out in accordance with the guidelines outlined in the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement [16].
This review included human clinical studies and was conducted by searching relevant literature in PubMed/ MEDLINE, Google Scholar, and Cochrane Library from 2018 to August 2024. The search focused on English-language publications and utilized relevant keywords. The following search string was applied: (“atrophic maxilla” OR “atrophic posterior maxilla “AND “sinus lift “OR” sinus floor elevation” OR “maxillary sinus augmentation”) OR (“lateral window” OR “maxillary sinus grafting” OR “lateral approach” OR “sinus graft” AND “biomaterials” OR “histology”) OR (“maxillary sinus lift” OR “sinus lift” AND “bone substitutes” OR “histomorphometry”).
Study selection criteria included clinical trials focused on maxillary sinus floor augmentation using various biomaterials, requiring histological and histomorphometric analyses of the biomaterials (Table 1).
3. RESULTS
A comprehensive literature search resulted in the identification of 3285 articles. After screening for duplicates and applying pre-defined inclusion criteria, seven articles were deemed suitable for inclusion in this review. The selection process is detailed in Fig. (1).
3.1. Biomaterials for Maxillary Sinus Floor Augmentation
A wide variety of biomaterials have been used, either alone or in combination with autografts, in maxillary sinus floor augmentation [16-18].
Among osteoconductive materials, allografts (such as fresh-frozen bone, freeze-dried bone, and demineralized freeze-dried bone), xenografts (from bovine, equine, or porcine sources), and alloplastic materials (including various combinations of calcium phosphate, bioactive glasses, and polymers) have been recognized in dental literature for their ability to promote bone formation. Moreover, several studies have indicated that these biomaterials may not negatively affect clinical outcomes or implant survival when compared to autogenous bone [6].
| Study | Year of Publication | Number of Patients | Maxillary Sinus Floor Augmentation | Type of Grafting Material | Length of Observation Period (months) | Number of Biopsies | TBV | Non-mineralized Tissue | Residual Graft | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean SD | Mean SD | Mean SD | |||||||||||||
| Correia et al. [29] | 2021 | 12 | 12 | porcine xenograft | 6 months | 10 | 56.01± 2.86% | 43.99± 2.86% | N/A | ||||||
| 12 | Autologous bone graft | 57.31± 2.91% | 42.69± 2.91% | ||||||||||||
| La Monaca et al. [43] | 2018 | 6 | 1 | mineralized solventdehydrated bone allograft |
6 months | 6 | 20.1% | 57.5% | 22.4% | ||||||
| 1 | freeze-dried mineralized bone allograft |
32.1% | 47.8% | 20.1% | |||||||||||
| 1 | anorganic bovine bone |
||||||||||||||
| 16.1% | 46.7% | 37.2% | |||||||||||||
| 1 | equine-derived bone | 22.8% | 47.1% | 30.1% | |||||||||||
| 1 | synthetic micro-macroporous biphasic calcium-phosphate block consisting of 70% betatricalcium phosphate and 30% hydroxyapatite |
20.3% | 41.8% | 37.9% | |||||||||||
| 21.4% | 53.3% | 25.3% | |||||||||||||
| 1 | bioapatite-collagen | ||||||||||||||
| Lahoud et al. [44] | 2022 | 20 | 21 | Puros allograft mixture of 50% Cortical and 50% Cancelous |
6 months | 21 | 30.32 ±3.659% | - | 18.0657± 3.092% |
||||||
| Molnár et al. [56] | 2022 | 40 | 40 | particulate bovine xenograft with grain size between | 6 months | 29 | 27.8± 11.2% | 39.2±9.0% | 32.9±6.3% | ||||||
| - | - | - | - | 0.5—1.0 mm without collagen membrane | - | - | 30.3±4.5% | 37.9 ±8.5% | 31.8 ±8.8% | ||||||
| Galindo-Moreno et al. [57] | 2022 | 10 | 20 | porcine bone mineral mixed with autogenous bone at a 20:80 ratio | 6 months | 20 | 32.51± 14.76% | 36.28 ± 13.43% | 31.21 ± 19.61% | ||||||
| anorganic bovine bone mixed with autogenous bone at a 20:80 ratio | 29.24 ± 10.82% | 39.76 ± 16.10% | 31.00 ± 17.29% | ||||||||||||
| Kraus, Riccardo et al. [74] | 2020 | 51 | 51 | biphasic calcium phosphate (BCP), HA/TCP 10:90 | 6 months | 62 | 35.9% | 38.1% | 25.3% | ||||||
| deproteinized bovine bone mineral (DBBM) | 35.4% | 18.2% | 45.9% | ||||||||||||
| Rodrigo Dos Santos et al. [75] | 2020 | 40 | 40 | autogenous bone graft (AB) | - | - |
Pristine bone (%SD) |
Intermediate (%SD) |
Apical (%SD) |
Pristine bone (%SD) |
Intermediate (%SD) |
Apical (%SD) |
Pristine bone (%SD) |
Intermediate (%SD) |
Apical (%SD) |
| bioactive glass (BG) | 37.8±16.9 | 38.1±21.4 | 44.5±18.6 | 57.6±16.3 | 58.8±20.9 | 54.0±16.5 | 1.5 | 0.5 | 2.5 | ||||||
| bioactive glass added to autogenous bone graft (BG + AB) 1:1 |
|||||||||||||||
| 43.6±4.7 | 37.3±10.9 | 49.3±13.2 | 56.6±6.5 | 63.4±11.0 | 49.3±13.2 | 0 | 0 | 0 | |||||||
| 39.0±15.8 | 34.8±14.5 | 36.8±14.5 | 60.3±11.9 | 62.1±14.5 | 58.5±13.6 | 1.5 | 1 | 2.5 | |||||||
| Bio-Oss (BO) |
|||||||||||||||
| 33.4±12.6 | 32.5±10.8 | 34.3±12.7 | 38.3±9.5 | 40.9±7.1 | 41.0±7.2 | 36.0 | 26.5 | 25.5 | |||||||
| Bio-Oss added to autogenous bone graft (BO + AB) 1:1 |
|||||||||||||||
| 32.8±11.5 | 36.1±16.0 | 27.8±19.8 | 46.9±14.5 | 40.9±21.9 | 43.4±15.9 | 22.5 | 24.5 | 36.0 | |||||||
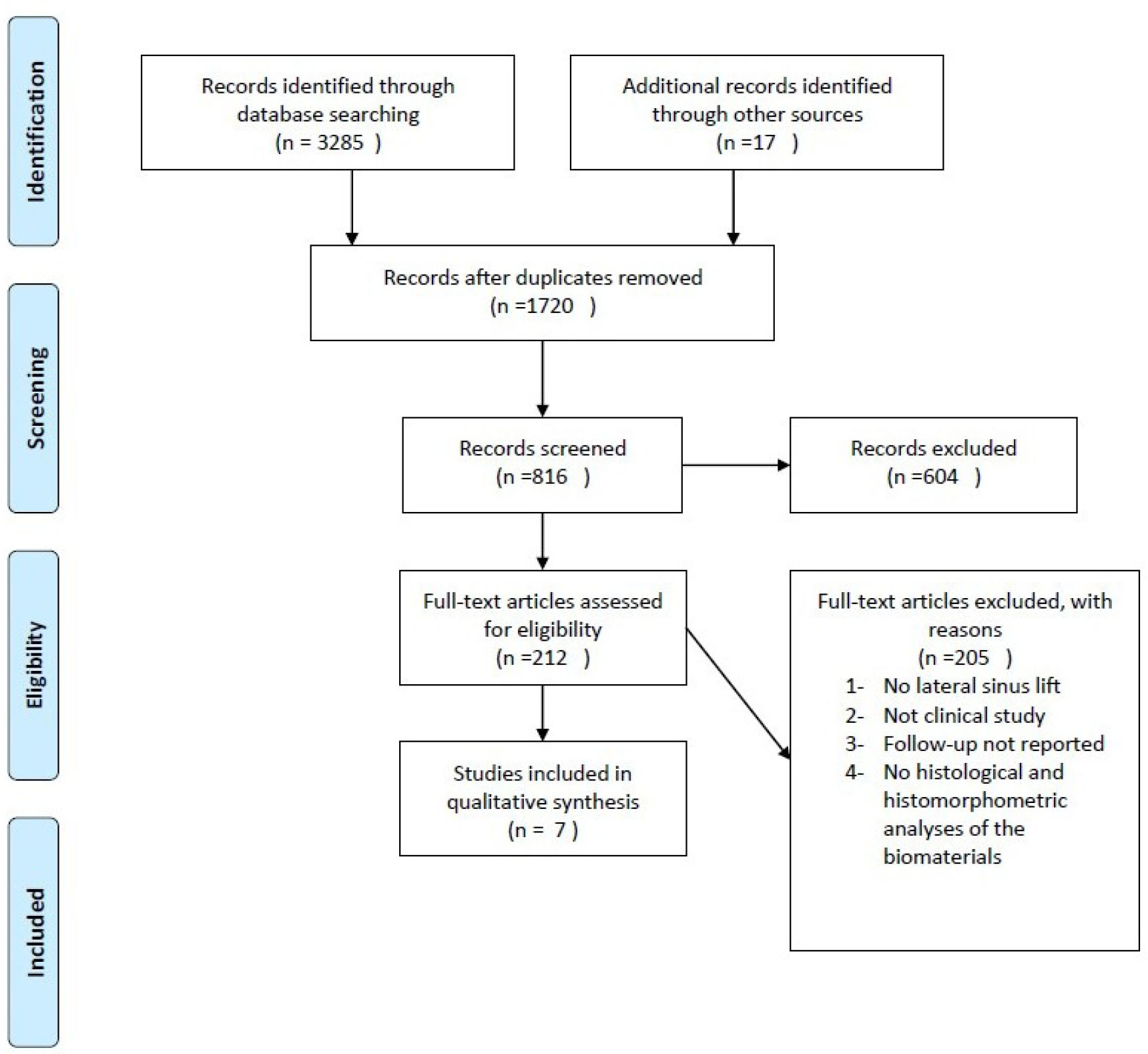
The PRISMA flow diagram of the selection process.
Deproteinized bovine bone mineral (DBBM) is one of the most widely used and extensively documented bone substitute materials in dental surgery [4]. The combination of DBBM with resorbable collagen membranes has proven to be effective for bone augmentation, particularly in scenarios involving dental implant placement [19]. DBBM has also been thoroughly studied in sinus floor elevation procedures, showing excellent long-term outcomes. However, despite its strong osteoconductive properties, DBBM lacks osteoinductive potential [20]. Alternatively, alloplastic materials can be utilized [21].
Biphasic calcium phosphate (BCP), composed of hydroxyapatite (HA) and β-tricalcium phosphate (β-TCP) in a 60:40 ratio, demonstrated comparable bone alterations to standard graft materials. However, these allografts exhibited a higher resorption rate during new bone formation [16]. Despite this, the implants placed afterward showed excellent survival and success rates [22].
A novel second-generation biphasic calcium phosphate (BCP) material, characterized by a 10:90 HA/TCP ratio and controlled microporosity, holds promise for improved osteoconductivity [23]. This material has demonstrated high biocompatibility, osteoconductivity [24, 25], and potential for osteoinduction [26]. While these findings are encouraging, human data remains limited.
3.2. Autogenous Bone Grafts
Autologous bone grafts can be harvested from both intraoral and extraoral donor sites and can be composed of cortical, medullary, or corticomedullary bone. These grafts can be used in fresh or frozen form. They are frequently employed in cases of severe bone atrophy to achieve sufficient ridge augmentation for dental implant placement. Autologous bone grafts are unique among bone substitutes in that they possess osteogenic potential, maintaining viable cells from the donor site to the grafting site when properly treated, and sharing the same biological origin as the recipient. This ensures no risk of rejection and strongly promotes neoangiogenesis and bone regeneration, providing solid physical support for membranes and flaps. However, the limitations of autologous bone grafts include their limited availability and the requirement for a second surgery, which increases morbidity. Additionally, there may be residual disability at the donor site, and there is a risk of complications, such as seizures and osteitis or osteomyelitis [27].
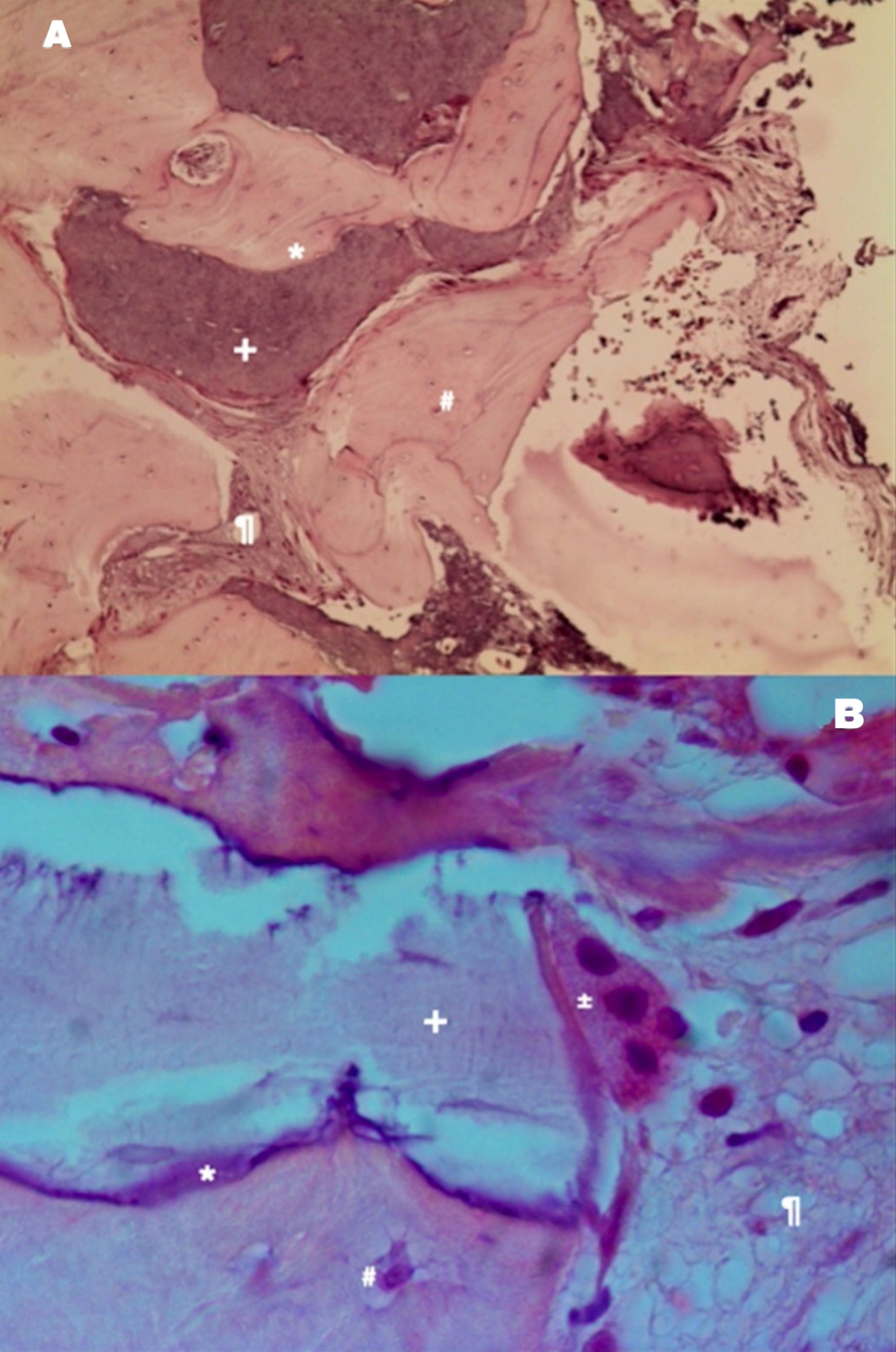
Autologous graft shows the bone vitality represented by osteoclastic reabsorption of the autologous bone graft and apposition of new bone with osteoblasts. (A) Example of autologous bone graft—H&E coloration—200×. (B) Autologous bone graft sample—H&E staining—400 [30]. Note: * immature bone, + autologous bone graft, # osteocyte, % xenograft, ¶ soft tissue, @ osteoblast, ± osteoclast, & Howship lacunae.
From a histological perspective, autologous bone exhibits remarkable osteoconductive properties. The majority of grafted particles are surrounded by newly formed bone, characterized by a direct and tight contact without any intervening fibrous connective tissue. The absence of inflammatory cells and multinuclear giant cells in the vicinity of the autologous bone grafts suggests excellent biocompatibility. Comparative studies on other bone substitute biomaterials (BSBs) consistently demonstrate that autologous bone [AB] promotes the greatest volume of newly formed bone, highlighting its potential for bone regeneration [28].
In the study by Francisco Correia [29], autograft was assessed in maxillary sinus floor augmentation, where autologous bone was harvested from the mandibular branch in 83.3% of cases and from the chin in only 16.7% of cases. Radiological findings revealed that the tissue height gains presented values ranging from 3.7 to 12.5 mm for the autologous graft, allowing the placement of dental implants with lengths ranging from 9 to 11 mm. Histological examination revealed various stages of bone remodeling, with the autologous bone integrated into the newly formed bone tissue and partially surrounded by vascularized tissue. No signs of inflammation or infection were evident. The histological analysis revealed only a few remaining particles that were fully integrated with the newly formed vital bone (Fig. 2) [29]. The medullary spaces, between the bone and graft particles, were filled with soft tissue rich in blood vessels.
3.3. Allografts
Despite its designation as the “gold standard” in bone regeneration, autologous bone grafting is limited by the finite availability of donor tissue and the risk of postoperative morbidity at the donor site. This has motivated researchers to investigate alternative, non-autologous sources for bone substitutes [30].
Allografts refer to the homologous bone, obtained from living or nonliving donors within the same species as the recipient, which undergoes a series of treatments and sterilization procedures before being stored in bone banks. These bone banks maintain a collection of homologous bone grafts in a variety of shapes and sizes, ready for surgical use [31]. Allogenic bone grafts come in various forms:
3.3.1. Fresh Frozen Bone (FFB)
Extracted and treated with low temperatures to minimize the risk of infection and reduce antigenicity.
3.4. Fresh Frozen Bone (FFB)
The preparation of fresh-frozen bone (FFB) is relatively straightforward; it is harvested, frozen at −80 °C, and stored in certified bone banks. Unlike other graft types, FFB does not require irradiation, lyophilization, or demineralization prior to storage or use [32]. Despite being acellular, this material retains its osteoinductive and osteoconductive properties, similar to those of autologous bone, due to its mineral composition, which includes bone morphogenetic proteins (BMPs) [33, 34]. Despite evidence suggesting that the cryoprotectant dimethyl sulfoxide (DMSO) may facilitate the survival of osteoblasts, osteoclasts, osteocytes, and periosteal cells during freezing, this material cannot be considered an osteogenic biomaterial [35]. While concerns about viral transmission once limited the use of FFB, rigorous donor selection and pretreatment have drastically reduced this risk to near zero. The altered protein structure of the material also renders it non-antigenic [36, 37].
Studies have demonstrated that fresh frozen bone (FFB) integrates seamlessly with existing bone, exhibiting no distinction from newly formed bone. This integration is characterized by osteoblasts depositing osteoid, remodeled areas with mineralized matrices containing osteocytes, and the formation of new blood vessels. Importantly, FFB use in both postextraction alveolar socket preservation and maxillary sinus augmentation results in minimal inflammation [38].
3.5. Freeze-dried Bone Allograft (FDBA)
Fresh frozen demineralized bone allograft (FDBA) is typically harvested from live, healthy donors undergoing hip replacement surgery. The trabecular and/or cortical bone is collected under sterile conditions, washed, fragmented to varying sizes (500 µm to 5 mm), treated with ethanol to remove lipids, frozen in nitrogen, dehydrated, and finally reduced to smaller particles (250-750 µm) [39].
Dehydration during FDBA processing enhances long-term preservation and reduces antigenicity, while retaining both organic and inorganic components, including calcium and phosphate. While FDBA demonstrates comparable clinical outcomes to demineralized freeze-dried bone allograft (DFDBA), it lacks the osteoinductive potential attributed to BMPs present in DFDBA following commercial preparation. Interestingly, a histological study on monkeys revealed a trend towards faster and greater bone formation with FDBA compared to DFDBA [40].In contrast, a human study found significantly more vital bone tissue and fewer residual graft particles in sites grafted with DFDBA compared to FDBA at the time of dental implant placement [41].
3.6. Demineralized Freeze-dried Bone Allograft (DFDBA)
Demineralized freeze-dried bone allograft (DFDBA) is a type of bone tissue sourced from deceased donors within 24 hours of death. After careful extraction, the bone is processed in a dedicated bone bank. This process involves washing with hydrogen peroxide, delipidation with ethanol, heat treatment, lyophilization, sterilization with ethylene oxide or gamma rays, and finally, demineralization in hydrochloric acid [32]. The preparation of DFDBA involves the removal of the inorganic mineral phase of bone, preserving the organic matrix, which is potentially responsible for the biomaterial's osteoconductive properties [42].
In a clinical study conducted by La Monaca et al. in 2018 [43], the effectiveness of various bone substitute materials used as grafts for maxillary sinus augmentation was evaluated in human subjects following a healing period of six months. Histological and histomorphometric results concerning mineralized solvent-dehydrated bone revealed that, at low magnification, trabecular bone with large marrow spaces and biomaterial particles was visible (Fig. 3a) [43]. The biomaterial particles varied in size and were partially encircled by newly formed bone. This newly formed bone was characterized by large osteocyte lacunae and largely bridged the biomaterial particles (Fig. 3b) [43]. In certain areas, osteoblasts were observed actively depositing new bone directly onto the surface of the graft particles. Only a few inflammatory cells were detected in the marrow spaces. Histomorphometric analysis revealed that newly formed bone comprised 20.1%, marrow spaces accounted for 57.5%, and residual graft material accounted for 22.4%.
Freeze-dried mineralized bone allograft (FDBA) was also examined. At low power magnification, newly formed bone, accompanied by marrow spaces and residual biomaterial particles, was observed. In the peripheral area of the sample, preexisting bone with small remodeling zones was visible (Fig. 4a) [43]. At high power magnification, some fields revealed that the biomaterial particles were fully osseointegrated, with areas of bone neoformation also evident within the particles. Some biomaterial particles displayed irregular margins, indicative of a resorption process (Fig. 4b) [43]. Areas of bone neoformation were noted both in contact with the biomaterial particles and within the marrow spaces, where a few spindle cells were also identified. Histomorphometric analysis indicated that newly formed bone comprised 32.1%, marrow spaces accounted for 47.8%, and residual graft material made up 20.1%.
A clinical study by Lahoud et al. (2022) [44], aimed to evaluate, after a six-month healing period, the histological and histomorphometric results of mineralized bone allograft in lateral sinus augmentation procedures. Six months after allograft regeneration, biopsies revealed a blend of allograft particles and newly formed bone trabeculae (Fig. 5) [44]. Residual bone graft particles were encircled by newly formed trabecular bone and marrow spaces, with blood vessels consistently present. The intertrabecular spaces, well-vascularized, were occupied by connective tissue and bone marrow. No signs of acute or chronic inflammatory infiltrate or foreign body reaction were observed. High magnification revealed new lamellar bone with active osteocytes, arranged in a typical lamellar pattern around Haversian canals, which is a characteristic of mature secondary cancellous bone (Figs. 6-7) [44]. At the periphery of the grafted bone, the mineralization front between bone particles and newly formed bone exhibited a smooth transition, indicating good integration.
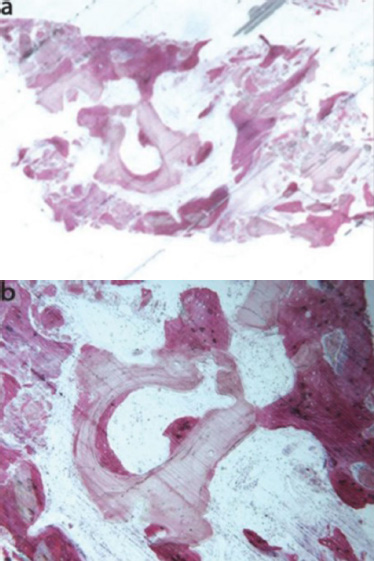
Mineralized solvent-dehydrated bone (toluidine blue and acid fuchsin): (a) Trabecular bone with large marrow spaces and biomaterial particles was observed (original magnification 12X); (b) Biomaterial particles (varying sizes) were partially embedded in bone with large osteocyte lacunae (40X) [44].
A high concentration of osteoblastic/osteoclastic activity indicated active bone remodeling, with numerous osteocytes in lacunae (Fig. 8) [44]. The newly formed bone displayed osteocytic lacunae filled with viable osteocytes and consisted predominantly of woven bone (Fig. 9) [44]. Osteoblastic cells appeared to be closely associated with the osteoid matrix surrounding the new bone. In contrast, the central areas of the residual particles showed empty osteocytic lacunae and a lamellar pattern.
3.7. Xenografts
Animal-derived bone substitutes have shown comparable osteoconductive properties to human bone grafts in clinical settings. However, their complete resorption and replacement by new bone remain poorly understood [45]. It has been observed that biomaterials that resist resorption may hinder proper bone regeneration due to their limited ability to stimulate new bone formation compared to natural bone during the remodeling process [46].
Freeze-dried mineralized bone allograft (toluidine blue and acid fuchsin): (a) Newly formed bone with marrow spaces and particles of residual biomaterial was present. In a marginal portion of the sample, preexisting bone with small remodeling areas could be observed (original magnification 12X); (b) The biomaterial particles, showing areas of bone neoformation in their inner part, could be observed. Some of the biomaterial particles showed irregular margins, typical of a resorption process (original magnification 40X) [44].
Heterologous bone substitutes offer a versatile solution for bone grafting, ridge augmentation, sinus elevation, and large bone defect correction, either alone or in combination with other materials. Ideally, they should mimic the regenerative properties of autologous bone while avoiding the need for additional surgery. Additionally, they should be biologically safe, possess osteogenic, osteoinductive, and angiogenic capabilities, have a long shelf life, be readily available in various sizes, and be cost-effective [47].
Numerous heterologous biomaterials have been proposed for clinical use in oral surgery, with bovine-derived bone substitutes emerging as a prominent subject of investigation. In vitro and clinical studies have consistently demonstrated the osteoconductive nature of these biomaterials, characterized by their ability to support the deposition of mineralized extracellular matrix by differentiated stem cells and their capacity to integrate seamlessly with host bone [48, 49].
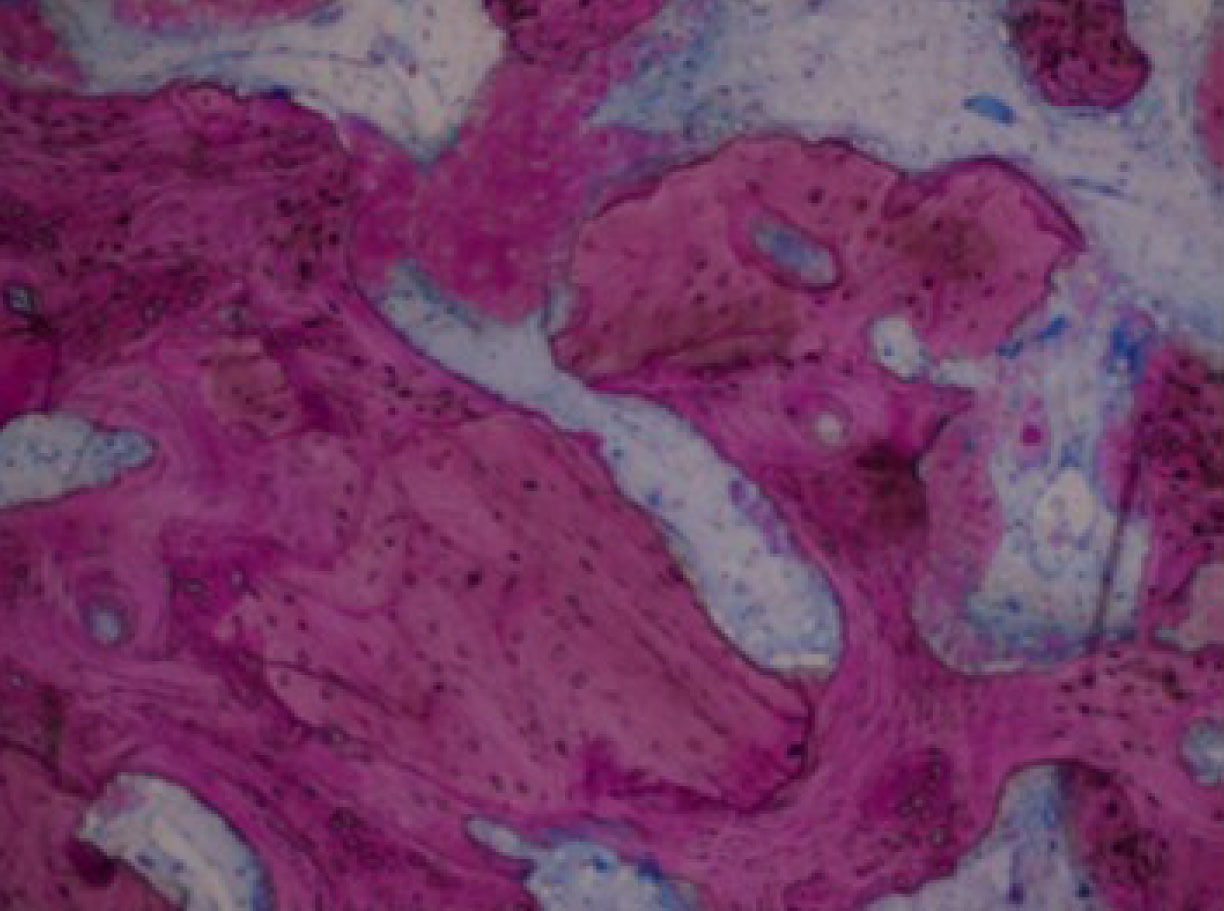
Biopsy showing the mixture made by allograft residual particles and newly formed bone [45].
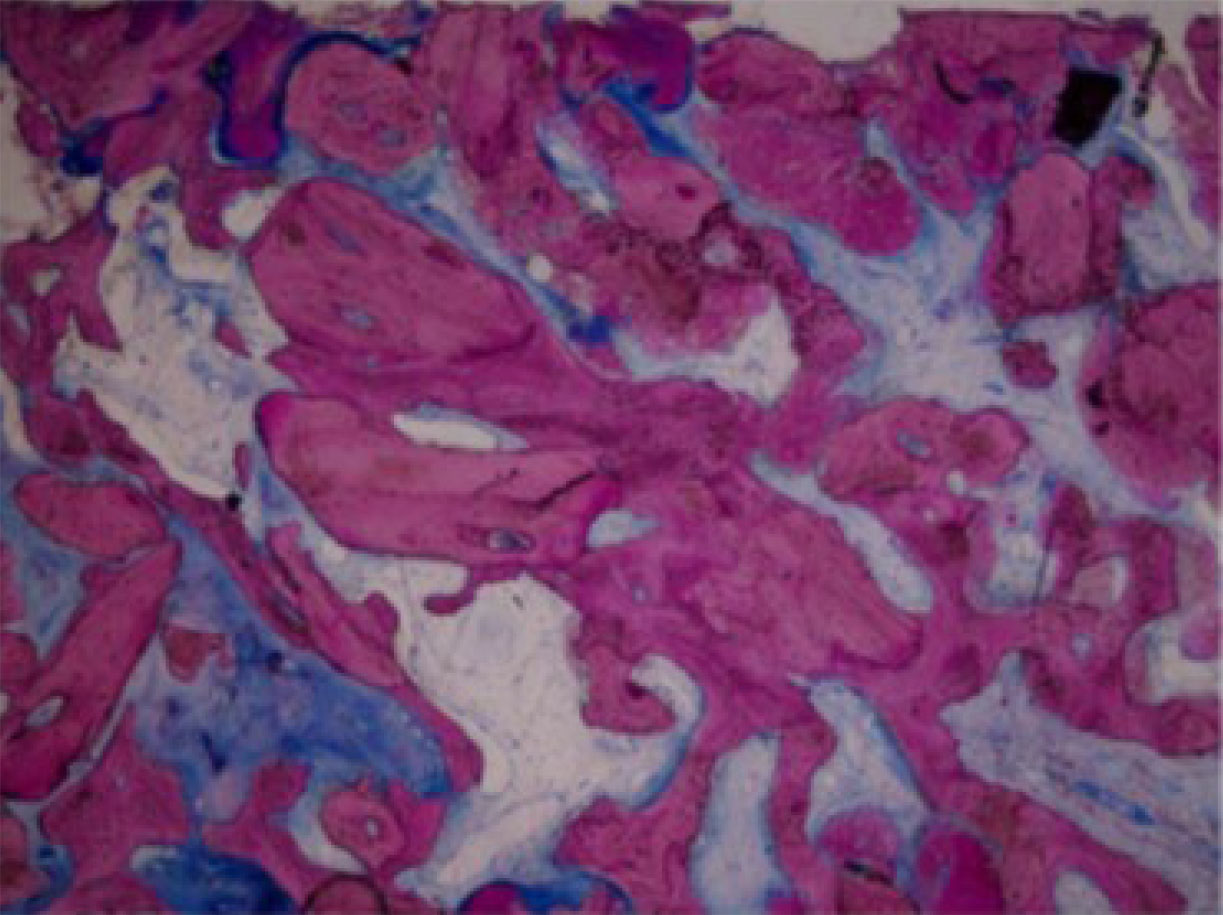
Low-power view (× 40) of numerous particles of Puros (P) incorporated with the newly formed bone (NB) [45].
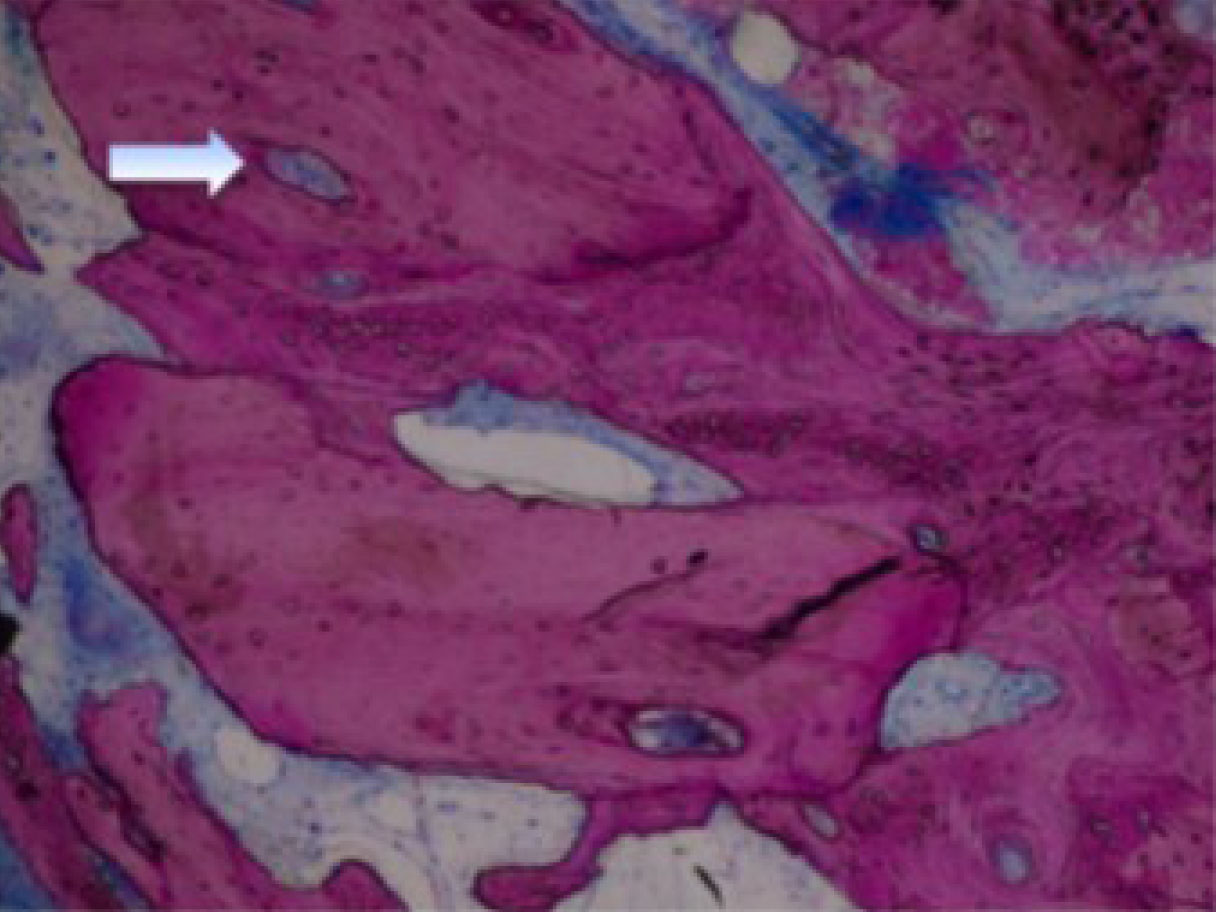
High-power view (× 100) of Puros allograft (P) in contact with new bone (NB) and osteoblasts (OB) on the mineralization front. The Haversian system (HS) is in the center of the allograft residual particles [45].
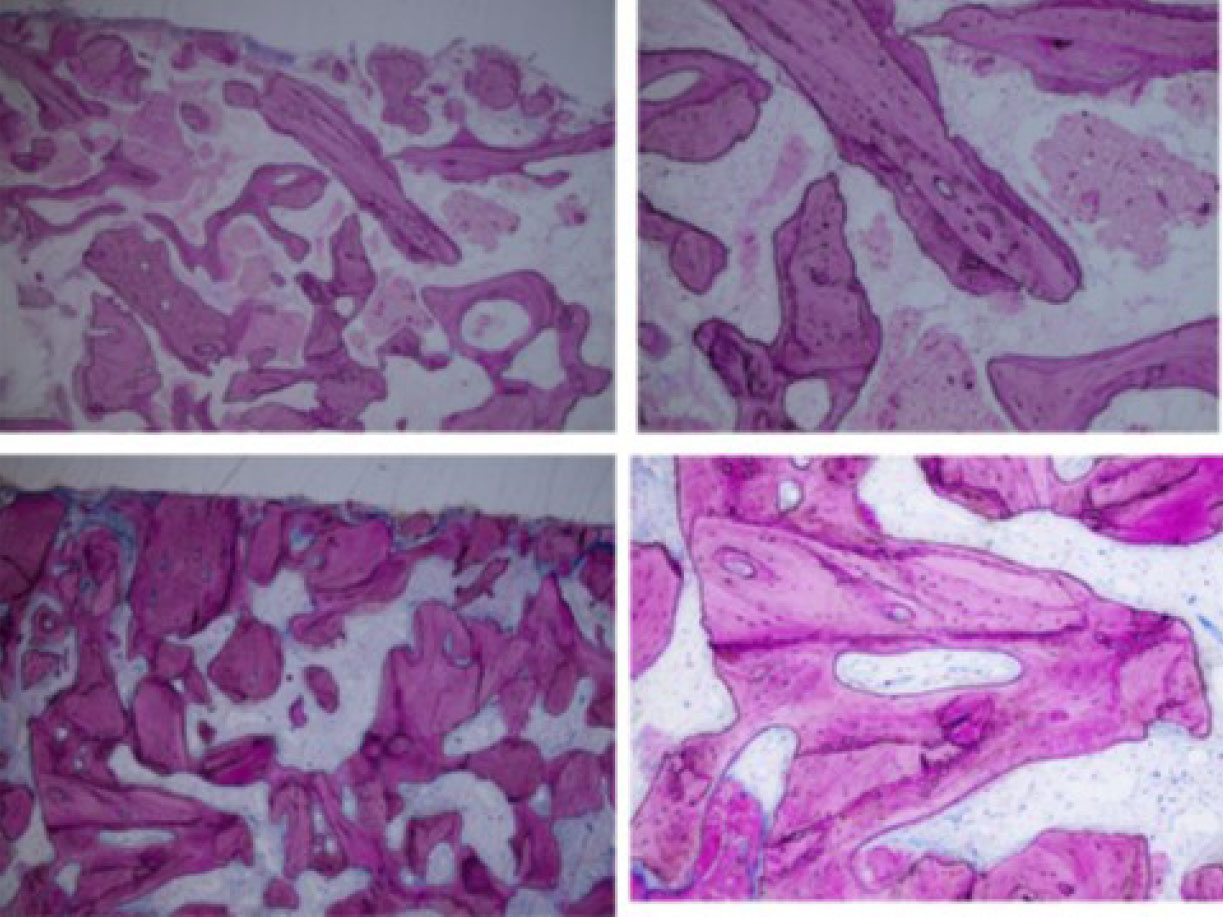
Highly cellular bone followed the outline of the grafted particles in direct contact in multiple pictures of biopsies (low and high-power view) [45].
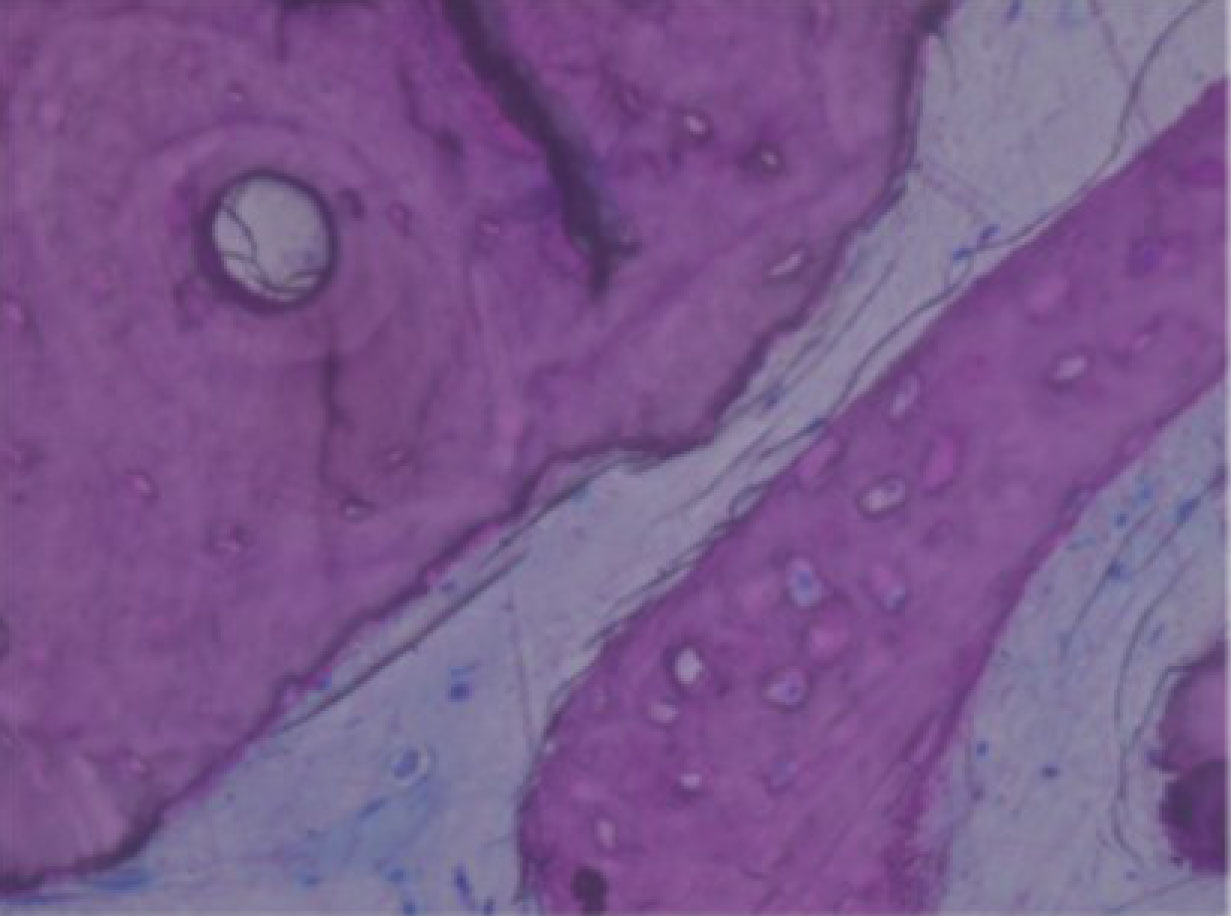
De novo bone with osteocytic lacunae filled with viable osteocytes (arrows) [45].
Unfortunately, these biomaterial granules often exhibit slow or incomplete resorption, resulting in new bone forming around them instead of being fully incorporated into the natural bone remodeling process [50].
The specific processing of bovine-derived bone substitutes, including high-temperature deproteinization and sterilization, may contribute to their slower resorption rates observed in vivo. While these treatments are essential for eliminating potential immunologic and allergenic risks, they also modify the mineral structure of the bone hydroxyapatite (HA), leading to reduced resorption rate.
The removal of organic components through prolonged heat treatment is crucial to prevent adverse reactions and cross-infection. However, this process can alter the HA structure, ultimately reducing the biomaterial's ability to be naturally broken down and replaced by new bone. Furthermore, recent concerns about prion disease transsmission have spurred research into alternative sources for bone substitutes [51].
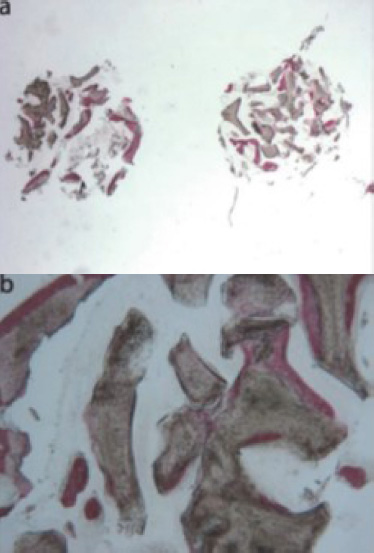
Anorganic bovine bone (toluidine blue and acid fuchsin): (a) the specimen appeared to be constituted by two separate fragments, where several particles of residual biomaterial were evident (original magnification 12X); (b) the areas of bone neoformation in tight contact with the biomaterial surface were present. In some fields, new bone formation inside the biomaterial particles could be observed (original magnification 40X) [44].
Recent studies suggest that a biological deantigenation process, using digestive enzymes at 37°C for 7 days, can effectively remove organic components while preserving the biomaterial's ability to be reabsorbed by the host [52]. To address the limitations of bovine-derived bone substitutes, equine-derived alternatives have emerged as promising options. Equine bone has demonstrated the ability to stimulate bone cell differentiation, undergo resorption by bone-degrading cells (osteoclasts) in laboratory settings, and has shown successful application in augmenting the mandible [53, 54].
While light microscopy analysis reveals limited evidence of particle resorption, porcine-derived bone substitutes demonstrate efficacy in inducing osteogenesis within guided bone regeneration protocols [55].
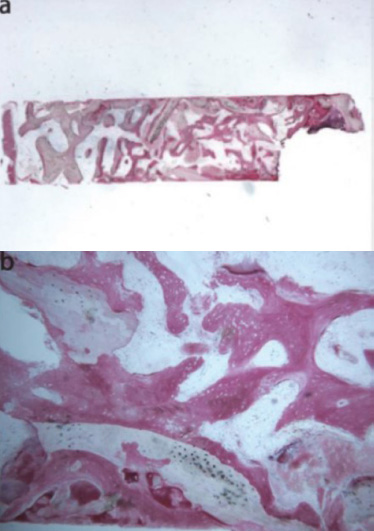
A 2018 clinical study led by La Monaca et al. [43] compared the efficacy of various bone graft materials, including heterologous substitutes, in maxillary sinus augmentation. The study tracked patient progress over a six-month healing period. Histological and histomorphometric analysis of anorganic bovine bone (ABB) revealed that at low magnification, two distinct fragments were observed, each containing several residual biomaterial particles (Fig. 10a) [43]. High magnification showed areas of bone neoformation in close proximity to the biomaterial surface (Fig. 10b) [43]. This newly formed bone exhibited large osteocyte lacunae, which is a characteristic of young bone. In some areas, new bone formation was observed within the biomaterial particles. Histomorphometry revealed that the newly formed bone comprised 16.1% of the specimen, marrow spaces accounted for 46.7%, and the residual biomaterial made up 37.2%.
Moreover, examination of equine-derived bone (EB) revealed new bone formation surrounding biomaterial particles located in the apical portion of the biopsy (Fig. 11a) [43]. Osteoblasts were observed actively depositing bone directly on the particle surface (Fig. 11b) [43]. The absence of inflammatory cells and multinucleated giant cells around the biomaterial suggests good biocompatibility. Histomorphometry confirmed the presence of newly formed bone (22.8%), marrow spaces (47.1%), and residual graft material (30.1%).
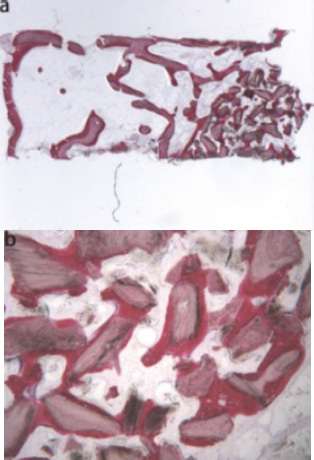
Equine-derived bone (toluidine blue and acid fuchsin): (a) Trabecular bone (12X) showed large marrow spaces and biomaterial particles within newly formed bone apically; (b) the bone was in close contact with the granules and in some areas, osteoblasts were observed depositing bone directly on the particle surfaces (original magnification 40X) [44].
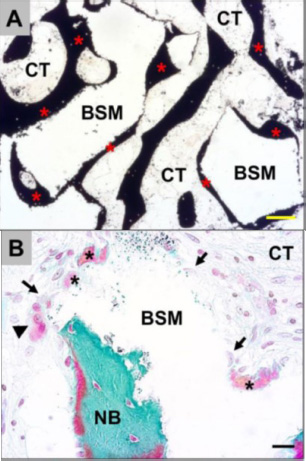
Exemplary histological images showing the integration behavior of the xenogeneic bone substitute material (BSM) that was observed in both study groups without any differences. (A): The material granules were mainly embedded within newly grown bone matrix (red asterisks). CT = connective tissue (von Kossa staining, × 100 magnification, scalebar = 50 μm). (B and C): New bone formation (black asterisks) was regularly observed at the BSM surfaces associated with active osteoblasts (blue arrow in C), indicating that the bone growth process was still in progress. At the surface areas that were covered by connective tissue (CT), mainly macrophages (black arrows) and single multinucleated giant cells (black arrowheads) were detected. Interestingly, osteoclasts (yellow arrowhead) were regularly found in the direct neighborhood of these areas, and their cellular arrangement did not significantly differ from that of the material-associated giant cells. NB = newly formed bone tissue, blood vessels = red arrows (Masson-Goldner staining, × 400 magnification, scale bars = 10 μm) [57].
A randomized controlled clinical study by Molnár et al. [56], investigated bone formation in lateral maxillary sinus augmentation using bovine bone substitute. The study compared two techniques: repositioning the bony wall or using a collagen membrane. Histological analysis revealed comparable tissue reactions to xenogeneic bone substitute material (BSM). New bone formation was observed on most bovine bone substitute granule surfaces (Fig. 12A) [56], with ongoing bone growth indicated by the presence of osteoblasts and multinucleated cells in connective tissue areas (Fig. 12B and C) [56]. While macrophages and single multinucleated giant cells were present, no inflammatory processes were detected in the intergranular connective tissue. Histomorphometric analysis revealed comparable tissue fractions in both study groups, with no significant interindividual variation. The mean percentage of newly formed bone was 27.8 ± 11.2% in the bony wall (BW) group and 30.3 ± 4.5% in the collagen membrane (CM) group. Similarly, the mean percentage of remaining xenogeneic bone substitute material was 32.9 ± 6.3% in the BW group and 31.8 ± 8.8% in the CM group. Connective tissue fractions averaged 39.2 ± 9.0% in the BW group and 37.9 ± 8.5% in the CM group. These histomorphometric findings did not demonstrate any significant differences between the groups.
In 2022, a randomized controlled trial by Moreno [57] compared the effectiveness of two xenografts (porcine bone mineral and anorganic bovine bone) for maxillary sinus floor augmentation, using a 20:80 mix of each xenograft with autogenous bone graft collected from the sinus access window. Histological analysis indicated that both pristine and grafted areas displayed similar characteristics in the two groups, with no statistically significant differences in any tissue sections (Fig. 13) [57]. New trabecular bone formation adjacent to the particles of both biomaterials was observed in the grafted areas of the biopsies (Figs. 14a, b and 15a-d) [57]. The non-mineralized tissue exhibited a comparable proportion of mesenchymal stromal cells and inflammatory infiltrate in biopsies from both biomaterials. The number of osteoid lines was significantly higher in areas grafted with autogenous cortical bone combined with anorganic bovine bone compared to the pristine bone in those biopsies. Furthermore, the areas grafted with autogenous cortical bone combined with anorganic bovine bone had more osteoid lines than those grafted with autogenous cortical bone combined with porcine bone mineral.
3.8. Alloplasts
A viable alternative to autologous bone grafts is synthetic biomaterials that mimic native bone tissue and serve as 3D scaffolds for cell growth and bone formation. Alloplastic substitutes, including calcium phosphate, calcium sulfate, bioactive glasses, and polymers, exhibit excellent osteoconductive properties, supporting osteogenic cell migration, in vitro growth, and mineralized extracellular matrix deposition by osteoblasts [58].
The design of bone substitutes relies on their ability to resemble natural bone properties, providing a 3D scaffold for cell ingrowth and tissue formation. Key factors for successful bone healing include the biomaterial's chemical composition, geometry, structure, and mechanical properties, as well as its resorption capability, which enables replacement by new bone. Strategies to enhance bone growth within the scaffold include surface nanotopography, biomimetic materials, mineralized layers, and bioreactors, all aiming to replicate the natural environment for bone cell development [30].
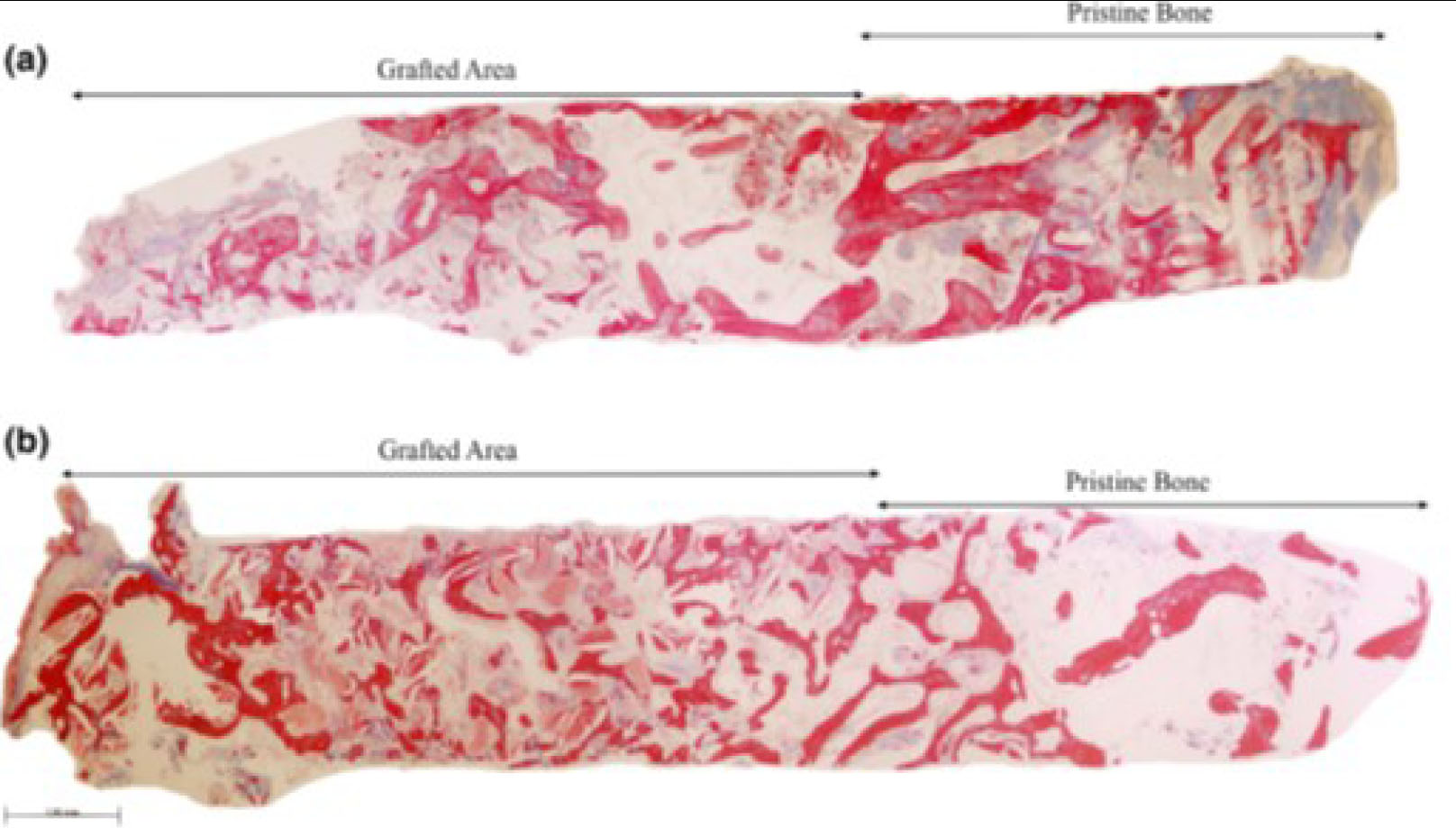
Representative panoramic microphotograph of biopsies from the (a) ACB+PBM and the (b) ACB+ABB groups, including pristine bone and grafted areas. Masson trichrome staining. Bar scale: 100 μm [58].
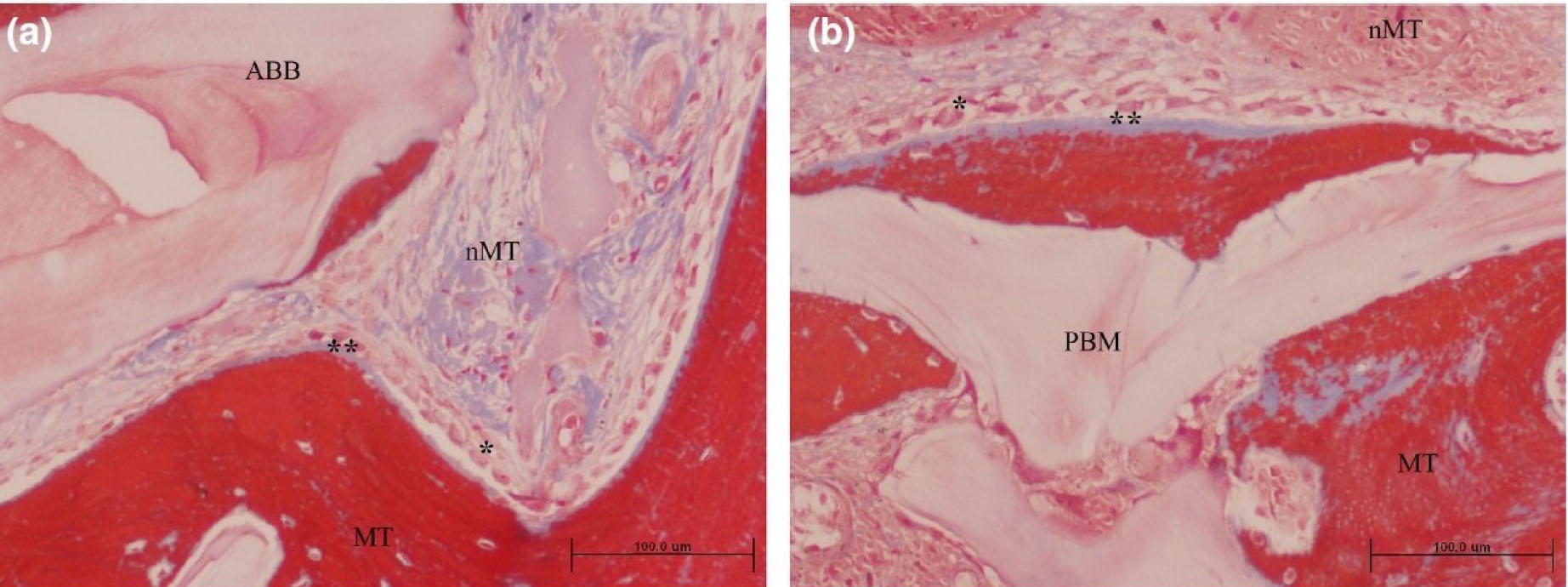
Grafted area with active osteogenesis as determined by the presence of osteoblastic (*) and osteoid lines (**) in (a) ACB+ABB and (b) ACB+PBM groups. Masson trichrome staining. Original magnification 20×; bar scale: 100 μm. ABB, anorganic bovine bone; MT, mineralized tissue; nMT, non-mineralized tissue; PBM, porcine bone mineral [58].
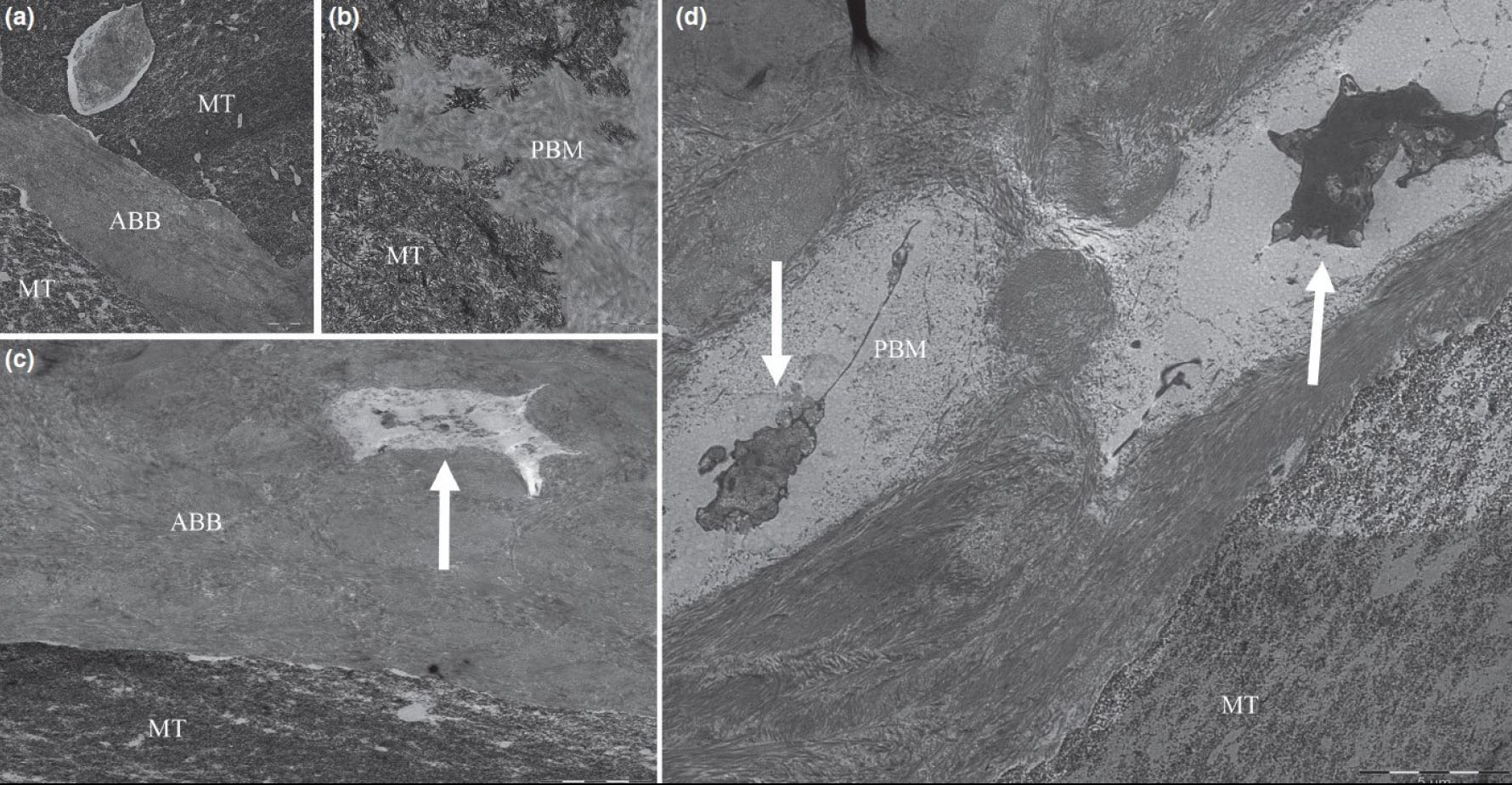
Transmission electron microscopy images showing close connection between porcine bone mineral (PBM) (a) and anorganic bovine bone (ABB) (b) particles and newly mineralized tissue (MT). Colonization by cells in PBM (c) and ABB (d) particles can also be observed (white arrows). Bar scale: 1 and 5 μm [58].
Among calcium phosphate-based materials, β-tricalcium phosphate (β-TCP) and hydroxyapatite (HA) are considered the most suitable ceramics for bone reconstruction. Studies using 2D and 3D computed tomography, along with histologic and histomorphometric analyses, have demonstrated that β-TCP promotes new bone formation comparable to that of autologous bone in maxillary sinus augmentation [59]. New bone formation was noted with the use of β-TCP in post-extraction alveolar sockets before dental implant placement. The grafted material demonstrated good integration with the newly formed bone, accompanied by the expression of osteonectin, a protein associated with osteoblast differentiation and metabolism [60].
Studies have reported that the chemical dissolution of β-TCP particles lowers local pH, promoting bone tissue regeneration [61]. Hydroxyapatite (HA) is a biocompatible and bioactive material, either naturally derived or synthetically produced. It can be categorized based on porosity as dense, microporous, or macroporous, and by structure as crystalline or amorphous [62].
An experimental study conducted on sheep tested nonresorbable porous HA as a bone substitute material for maxillary sinus augmentation with dental implants. Bone-to-implant contact was similar to that of autologous bone grafts. However, due to its limited resorption, HA is recommended for use alongside autologous bone, rather than as the sole material [63].
A study by Götz et al. [64], on human jaw biopsies found that a commercially available porous nanocrystalline HA exhibited osteoconductive and biomimetic properties, with the ability to be fully resorbed and integrate into the natural bone remodeling process during healing.
The nanoporosity of the biomaterial enables the absorption of bone-specific molecules and growth factors, like alkaline phosphatase, BMP-2, collagen type I, osteocalcin, and osteopontin, facilitating osteoblast precursor recruitment and differentiation, along with osteoclast adhesion for graft replacement with mature bone. However, the inherent brittleness and low fracture toughness of HA can be enhanced by incorporating toughening materials, such as alumina, titanium, or carbon nanotubes (CNTs) [65-67].
Biphasic calcium phosphate ceramics are biocompatible, osteoconductive, degradable, and have adequate mechanical strength for bone reconstruction [68]. Their unique properties come from the balance between the less-soluble HA and the more-soluble TCP. Adjusting the HA/TCP ratio allows for customization of the ceramic's mechanical and biological performance to optimize bone and cartilage tissue formation [59].
In a study by Mangano et al. (2015), a custom 3D scaffold with programmed porosity and a 30/70 HA/TCP ratio was used to regenerate bone in sheep maxillary sinuses. The scaffold supported gradual tissue regeneration and degradation during healing. Histomorphometric and immunohistochemical analyses revealed that it was fully colonized by vascularized fibrous tissue with new bone forming mainly at the graft's periphery [69].
Calcium sulfate, a fully resorbable synthetic material, was previously used but absorbed too quickly, leading to replacement by connective tissue rather than bone. Today, heat-treated calcium hyposulfate is preferred for its slower resorption, which allows bone to form in its place [70].
A histologic study by Thomas et al. reported that calcium sulfate promotes predictable bone formation, aiding implant placement monitoring. Complete resorption of the material was observed after 9 months of healing [71].
A cutting-edge ceramic biomaterial called bioglass mimics some properties of biological tissues by forming a bioactive HA layer on its surface, facilitating bone and soft tissue integration. Most bioactive silicate glasses consist of 45% silicon dioxide and a 5:1 molar ratio of calcium oxide to phosphorus pentoxide. It has been observed that lower CaO/P2O5 ratios prevent bone bonding [72].
Ravarian et al. [73], conducted a histologic and histomorphometric study reporting that combining bioactive glass ceramics with autologous bone for maxillary sinus augmentation significantly accelerated bone regeneration. This hybrid material, comprising 80-90% bioglass and 10-20% autologous bone, promoted new bone formation within 6 months, compared to 12 months for bioglass alone. This synergistic effect not only enhances regeneration but also potentially overcomes the inherent mechanical weakness of bioglass.
La Monaca et al. conducted a clinical study in 2018 [43] to assess the effectiveness of synthetic micro-macroporous biphasic calcium-phosphate (HA-β-TCP 30/70) as a graft material for maxillary sinus augmentation. The study involved human subjects and assessed outcomes after a six-month healing period. The sample exhibited a new trabecular bone integrating with existing bone, surrounded by marrow spaces and residual biomaterial (Fig. 16a) [43]. The biomaterial showed seamless integration with new bone, with no gaps at the interface. While some areas showed evidence of graft resorption (Fig. 16b) [43], no inflammation or foreign body reaction was observed. Numerous blood vessels were present. Histomorphometric analysis revealed a composition of 20.3% new bone, 41.8% marrow, and 37.9% residual graft material.
In a randomized controlled clinical trial conducted by Kraus [74], a comparative analysis was performed on biphasic calcium phosphate (BCP) versus deproteinized bovine bone mineral (DBBM) for sinus floor elevation. The study found that the proportion of “new bone” formed was similar in both groups, with BCP at 35.9% and DBBM at 35.4% (p=0.845). However, the amount of remaining “bone grafting material” was significantly lower in the BCP group (25.3%) compared to the DBBM group (45.9%; p<0.001). Conversely, the BCP group showed a significantly higher mean proportion of non-mineralized tissue in biopsies at 38.1% compared to 18.2% in the DBBM group (p<0.001).

Synthetic micro-macroporous biphasic calcium-phosphate (HA-β-TCP 30/70) (toluidine blue and acid fuchsin): (a) Trabecular bone with marrow spaces and residual biomaterial, located in the apical portion of the sample, could be observed (original magnification 12X); (b) The residual biomaterial was surrounded by newly formed bone and no gaps were present at the bone biomaterial interface. In some fields, the graft seemed to undergo resorption. Many large blood vessels could be seen (original magnification 40X) [44].
A randomized clinical study by Pereira et al. [75] evaluated new bone and connective tissue formation, as well as the residual biomaterial, after maxillary sinus bone augmentation using bioactive glass combined with autogenous bone graft. The study revealed significant new bone formation, primarily characterized by lamellar organization and a greater presence of woven bone in the apical region. Connective tissue was found to contain cells and osteoblasts surrounding the bone matrix. The average new bone formation was 39.0% ± 15.8 in the pristine bone region, 34.8% ± 14.5 in the intermediate region, and 36.8% ± 14.5 in the apical region. For connective tissue, the mean was 60.3% ± 11.9 in the pristine bone, 62.1% ± 14.5 in the intermediate, and 58.5% ± 13.6 in the apical region. The remaining biomaterial had medians of 1.5 in the pristine bone, 1 in the intermediate, and 2.5 in the apical region.
CONCLUSION
This comprehensive review of the literature assessed the efficacy and biocompatibility of various bone substitute biomaterials employed in maxillary sinus augmentation procedures. The analysis of multiple studies revealed a consistent pattern; all assessed biomaterials demonstrated effectiveness and suitability for this challenging surgical application. This conclusion is supported by strong evidence of remarkable biocompatibility and significant osteoconductive properties.
Histological analyses consistently unveiled a positive and seamless interaction between the biomaterials and the host tissues. Specifically, osteoblastic cells were observed actively forming new bone tissue in direct contact with the surfaces of the biomaterials. This observation strongly suggests that the biomaterials not only serve as a scaffold for bone growth but also actively promote bone formation. The intimate contact and integration of biomaterials with the host tissue are further supported by the widespread finding that the majority of biomaterial particles become fully integrated within the newly formed bone trabeculae. This robust integration indicates a successful and seamless incorporation into the existing bone structure, minimizing the risk of long-term complications.
Importantly, the histological analyses revealed an absence of acute inflammatory infiltrates or any other signs of adverse tissue reactions. This lack of inflammatory response confirmed the excellent biocompatibility of the assessed materials, indicating that they are well-tolerated by the body and do not elicit any significant immunological reaction. Moreover, the biomaterials exhibit a gradual and controlled resorption process. This means that as new bone tissue forms, the biomaterial is progressively replaced, ultimately leaving behind a functionally integrated bone structure. The rate of resorption appears to be well-matched to the rate of bone formation, leading to optimal tissue regeneration.
Overall, the findings of this comprehensive review strongly support the clinical use of these bone substitute biomaterials in maxillary sinus augmentation. Their excellent biocompatibility, demonstrably high osteoconductive properties, and the favorable tissue integration observed across multiple studies solidify their position as valuable substitutes for achieving predictable and successful bone regeneration in this complex surgical environment. The limited follow-up duration in the included studies precludes a comprehensive assessment of long-term outcomes. Further investigation, incorporating extended follow-up periods and comparative analyses of diverse biomaterials, is warranted to optimize treatment strategies.
AUTHORS’ CONTRIBUTIONS
The authors confirm their contribution to the paper: N.S.: Study conception and design; N.S.: Data collection; N.S. and A.K.: Analysis and interpretation of results; N.S.: Draft manuscript. All authors reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| MSFA | = Maxillary Sinus Floor Augmentation |
| BIC | = Bone-to-Implant Contact |
| TBV | = Total Bone Volume |
| BAF | = Bone Area Fraction |
| RCTs | = Randomized Controlled Trials |
| DBBM | = Deproteinized Bovine Bone Mineral |
| BCP | = Biphasic Calcium Phosphate |
| HA | = hydroxyapatite |
| β-TCP | = Β-Tricalcium Phosphate |
| BSBs | = Bone Substitute Biomaterials |
| AB | = Autologous Bone |
| FFB | = Fresh Frozen Bone |
| FDBA | = Freeze-dried Bone Allograft |
| DFDBA | = Demineralized Freeze-dried Bone Allograft |
| BMPs | = Bone Morphogenetic Proteins |
| DMSO | = Dimethyl Sulfoxide |
| ABB | = Anorganic Bovine Bone |
| EB | = Equine-Derived Bone |
| BSM | = Bone Substitute Material |
| BW | = Bony Wall |
| CM | = Collagen Membrane |
| CNTs | = Carbon Nanotubes |
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article is available in the [Zenodo] at [zenodo.org], reference number [Doi: 10.5281/zenodo.16291264].
ACKNOWLEDGEMENTS
Declared none.


