All published articles of this journal are available on ScienceDirect.
Impact of 0.2% Hyaluronic Acid on TNF-α and TGF-β1 in Periodontitis Patients with Type 2 Diabetes Mellitus
Abstract
Introduction
Periodontitis is a chronic inflammatory disease that significantly impacts the health of patients with type 2 diabetes mellitus (T2DM) due to their impaired immune response and delayed healing capabilities. Hyaluronic acid (HA), known for its anti-inflammatory and regenerative properties, may influence cytokine activity crucial for periodontal tissue repair. Tumor Necrosis Factor-alpha (TNF-α) and Transforming Growth Factor-beta1 (TGF-β1) are inflammatory cytokines involved in the healing process.
Objective
This study aims to assess the effect of subgingival application of 0.2% HA gel on TNF-α and TGF-β1 levels during the healing process of periodontitis in patients with T2DM.
Methodology
A randomized clinical trial was conducted involving T2DM patients with diagnosed periodontitis. Participants received HA treatment alongside standard periodontal therapy. Gingival crevicular fluid (GCF) samples were collected before scaling and root planing (SRP) and at the 4-week follow-up to measure TNF-α and TGF-β1 levels using enzyme-linked immunosorbent assay (ELISA). Participants were randomly assigned to receive either 0.2% HA gel or a placebo gel, with a 1:1 allocation ratio. Identical packaging was used to maintain blinding. Following SRP, the applicator tip was carefully inserted subgingivally into the periodontal pocket, ensuring the gradual delivery of the gel until the pocket was filled, with gel visible at the gingival margin, indicating adequate treatment coverage.
Results
Biomolecular analysis revealed a substantial reduction in TNF-α levels (p<0.05) and an elevation in TGF-β1 levels (p<0.05) in both groups after 4 weeks of follow-up. Although the delta change analysis between initial assessment and follow-up at 4 weeks showed statistically insignificant results (p>0.05) for TNF-α and TGF-β1 levels in T2DM patients treated with 0.2% HA gel and placebo gel, a clear trend of TNF-α reduction and TGF-β1 elevation was observed.
Discussion
The results demonstrated that HA has a profound impact on the inflammatory and regenerative pathways in periodontal tissues of T2DM patients. The reduction in TNF-α levels suggests that HA effectively suppresses the chronic inflammatory response, which is crucial for managing periodontitis in T2DM patients. Concurrently, the increase in TGF-β1 levels indicates enhanced tissue repair and regeneration. This dual modulation by HA supports its use as an adjunctive therapy, providing both anti-inflammatory and regenerative benefits.
Conclusion
This study highlights the therapeutic potential of HA in managing periodontitis in T2DM patients by modulating key cytokines involved in inflammation and tissue regeneration. Further research is warranted to optimize dosing and administration protocols and to evaluate long-term outcomes.
1. INTRODUCTION
Periodontitis is a chronic inflammatory condition triggered by the presence of a bacterial biofilm, commonly known as dental plaque. This biofilm disrupts the supporting periodontal tissues, especially periodontal ligaments and alveolar bone. Evidence suggests a two-way interaction between periodontal disease and diabetes mellitus (DM) [1]. Type 2 diabetes mellitus (T2DM) is a significant risk factor for periodontitis. Diabetes can enhance the pathogenicity of periodontal microbiota and the inflammatory or immune response in the periodontium [2]. The coexistence of DM and periodontitis can lead to an impaired immune response and delayed healing capabilities, further complicating the management of both conditions. Managing periodontal disease may contribute to better glucose control. Increased levels of pro-inflammatory markers in the gingiva of patients with poorly controlled diabetes indicate a potential biological mechanism that could exacerbate periodontitis [1, 3].
Hyaluronic acid (HA) has been studied for its anti-inflammatory and regenerative properties, positioning it as a beneficial adjunct in the treatment of periodontitis, especially in patients with T2DM [4]. Research suggests that HA can positively influence cytokine activity, which is crucial for periodontal tissue repair. In T2DM patients with periodontitis, where inflammation and impaired healing are common, HA helps modulate the inflammatory response by reducing pro-inflammatory cytokines and modulating anti-inflammatory cytokines, such as Tumor Necrosis Factor-alpha (TNF-α) and Transforming Growth Factor-beta1 (TGF-β1). It also promotes periodontal tissue regeneration by stimulating osteogenic differentiation and enhancing the metabolic activity of periodontal ligament cells [5]. These properties make HA an effective component in managing periodontal disease in T2DM, improving clinical outcomes and aiding in tissue repair [6]. This study examines the impact of 0.2% hyaluronic acid gel on TNF-α and TGF-β1 levels in the healing process of periodontitis in patients with T2DM.
The pathogenesis of periodontitis involves a complex interplay between periodontal pathogens and the host immune response. Tumor Necrosis Factor-alpha is a key pro-inflammatory cytokine that plays a pivotal role in the immune response and the progression of periodontitis. TNF-α production is primarily triggered by activated macrophages, T cells, and other immune cells in response to bacterial components like lipopolysaccharides (LPS). This cytokine is a central regulator of inflammation, inducing the production of other pro-inflammatory cytokines, chemokines, and adhesion molecules that promote the recruitment and activation of immune cells at infection sites [7]. Elevated levels of TNF-α are often observed in the gingival crevicular fluid (GCF) of patients with periodontitis, correlating with disease severity and tissue destruction [8]. The role of TNF-α extends beyond localized periodontal inflammation, influencing systemic health conditions, particularly T2DM. Chronic hyperglycemia in T2DM is associated with an enhanced inflammatory state, marked by increased levels of TNF-α and other pro-inflammatory cytokines [9]. TNF-α contributes to insulin resistance by interfering with insulin signaling pathways, further complicating glycemic control [10]. The bidirectional relationship between periodontitis and DM is well-documented, with each condition potentially exacerbating the other. In T2DM patients, elevated TNF-α levels can aggravate periodontal inflammation, leading to more severe periodontal destruction and impaired healing [11]. Conversely, chronic periodontitis-induced inflammation can exacerbate systemic inflammation and impair glycemic control in T2DM, perpetuating a vicious cycle that complicates disease management [12].
Transforming growth factor-beta1 is a multifunctional cytokine involved in angiogenesis, immune suppression, extracellular matrix synthesis, apoptosis, and the inhibition of cell growth [13]. In periodontal disease, TGF-β1 can alternate between pro-inflammatory and anti-inflammatory roles depending on the host's immune response. Its pro-inflammatory properties include acting as a chemoattractant for neutrophils, monocytes, mast cells, and lymphocytes, as well as promoting the release of pro-inflammatory cytokines. Conversely, its anti-inflammatory role involves suppressing cell-mediated and humoral immune responses [14]. Studies have shown that TGF-β1 levels are higher in gingival tissues and gingival crevicular fluid in areas of inflammation compared to healthy sites [15]. Skaleric et al. observed elevated TGF-β1 levels in GCF samples from sites with deeper periodontal pockets [16]. Transforming growth factor-beta1 plays a key role in cell proliferation and differentiation, making it an essential cytokine for wound healing, tissue remodeling, and regeneration [13]. Previous studies have indicated that elevated TGF-β1 levels are linked to reduced periodontal inflammation, contributing to better clinical outcomes [17].
Effective management of periodontitis in patients with DM necessitates therapeutic strategies that not only address infection control but also regulate the inflammatory response and facilitate tissue repair. Hyaluronic acid, a naturally occurring glycosaminoglycan, has emerged as a promising adjunctive treatment in periodontal therapy due to its multifaceted biological properties. The therapeutic application of HA in periodontal disease is primarily due to its anti-inflammatory, anti-edematous, and wound-healing effects [18]. Studies have demonstrated that HA can effectively reduce the levels of TNF-α, a pro-inflammatory cytokine that is elevated in the periodontal tissues of patients with periodontitis. By reducing TNF-α levels, HA helps mitigate the inflammatory response, thereby preventing further tissue damage and promoting a more conducive environment for healing [5]. This anti-inflammatory action is particularly beneficial for patients with T2DM, who often exhibit elevated TNF-α levels due to chronic hyperglycemia and insulin resistance [19].
Research has shown that the adjunctive use of HA in periodontal therapy can lead to significant improvements in clinical, biomolecular, and microbiological parameters. For instance, a study by Madkour et al. [18] demonstrated that HA treatment reduced gingival inflammation and promoted tissue healing in patients with periodontitis. Similarly, Mohammad et al. reported that HA enhanced the effectiveness of scaling and root planing (SRP) by reducing inflammatory markers and supporting periodontal tissue regeneration [5]. Patients with periodontitis and T2DM often experience impaired healing processes. The administration of HA is expected to enhance healing, as evidenced by reduced inflammation.
Despite the known association between periodontitis and T2DM, the specific biomolecular mechanisms underlying the impact of HA on cytokine modulation in this population remain underexplored. While previous studies have shown that HA can reduce inflammatory markers in periodontal tissues, there is limited evidence regarding its effects on TNF-α and TGF-β1 levels in periodontitis patients with T2DM. The need for targeted therapeutic strategies that control infection, optimize cytokine balance, and promote tissue healing in diabetic individuals remains a significant research gap.
Understanding the molecular effects of HA on TNF-α and TGF-β1 levels in periodontitis patients with T2DM can provide valuable insights into its therapeutic potential. Given the bidirectional relationship between T2DM and periodontitis, a treatment that reduces inflammation and enhances tissue healing could have dual benefits in periodontal and systemic health management. This study aims to bridge this gap by investigating the specific role of HA in modulating cytokine levels in this high-risk population. This study aims to evaluate the impact of 0.2% HA gel on TNF-α levels in periodontitis patients with T2DM, assess the effect of HA on TGF-β1 levels and its potential role in periodontal tissue regeneration, and observe the overall influence of HA on the inflammatory response and healing process in periodontitis patients with T2DM, including comparisons between HA gel group and a placebo gel group.
2. MATERIALS AND METHODS
2.1. Study Design and Patient Selection
A double-blind, parallel, randomized controlled trial was carried out at the Periodontics Department Universitas Indonesia between March and June 2023, aimed at evaluating biomolecular levels in T2DM patients with periodontitis. Approval for the research procedure was granted by the Ethical Commission for Dental Research (KPEKG), Faculty of Dentistry, Universitas Indonesia, under approval number 130/Ethical Approval/FKGUI/XII/2022 and protocol reference number 090660722. In addition, the protocol was endorsed by the Strategic Initiative for Developing Capacity in Ethical Review (SIDCER) and the Forum for Ethical Review Committees in Asia and the Western Pacific (FERCAP). The study protocol received approval from the Ethics Committee, registered under ISRCTN49272905, and conducted in accordance with the Helsinki Declaration of 1975, as revised in 2013. Prior to enrollment, all participants were thoroughly briefed on the study procedures and signed a written informed consent form.
2.2. Inclusion Criteria and Exclusion Criteria
The study included 40- to 65-year-old male and female participants with periodontitis based on the 2017 criteria from the EFP [20, 21]. Participants had detectable interdental CAL at two or more non-adjacent teeth or buccal CAL of ≥3 mm with pocketing >3 mm at two or more teeth. Samples were obtained from a site with PPD with a range of 4 to 6 mm. Participants were required to have had an HbA1c test in the previous month, with results above 6.5% classified as T2DM. Additionally, participants needed medical clearance for SRP treatment and provided written informed consent to actively participate in the study.
The exclusion criteria were as follows: (1) regular use of medications influencing microbial activity (e.g., antibiotics, anti-inflammatory drugs); (2) history of bleeding disorders, mental health conditions, or acute infections; (3) receipt of periodontal therapy in the past six months; (4) allergy to HA gel; and (5) pregnancy or breastfeeding. Patients requiring antibiotic treatment during the study were excluded to prevent bias, as well as those who missed follow-up appointments.
2.3. Sample Size
The sample size was calculated using G*Power analysis software prior to study initiation [22]. Determination was based on Probing Pocket Depth (PPD), which was identified as the key outcome measure, with the assumption of a normal distribution. A power analysis was carried out using an effect size (Cohen’s d) of 0.9, which represents a large effect size as defined by Cohen, based on the reference described by Madkour et al. [18] To achieve 80% statistical power and detect a significant difference (p ≤ 0.05), a minimum of 9 subjects per group was required, resulting in a total of 18 participants. Participants were excluded from the study if any of the following conditions arose during the study period: pregnancy, use of anti-inflammatory or antibiotic medications, use of mouthwash, application of topical treatments near the study area, failure to attend the second visit, involvement in an accident, or voluntary withdrawal from the study.
2.4. Periodontal Examination and Measurements
Clinical periodontal assessments were performed by a calibrated examiner, with measurements taken at six sites per tooth. Probing pocket depth (PPD) was measured at baseline, prior to nonsurgical periodontal therapy, and at a 4-week follow-up utilizing a UNC-15 periodontal probe (Hu-Friedy, Chicago, IL, USA). Periodontal disease severity was evaluated based on CAL and PPD per the inclusion criteria, ensuring standardized assessments [20, 21]. The examiner underwent calibration to minimize variability, and measurements followed a predefined protocol.
2.5. Periodontal Therapy and Randomization
During the initial visit, all clinical parameters were assessed, GCF was collected, and SRP with gel application was performed. Prior to this, extensive intra-examiner calibration exercises were conducted to ensure the accuracy and consistency of the assessment process. A single operator (AAD) evaluated all clinical parameters and collected GCF from the selected teeth, which were sampled using three to four sterile paper points (size 25) positioned into the periodontal pockets for a duration of 30 seconds. Subsequently, a comprehensive subgingival and supragingival SRP procedure was performed on the entire mouth by a single operator (MM) utilizing an ultrasonic instrument. The randomization process, managed by the Faculty of Pharmacy, Universitas Indonesia, allocated subjects to either the control group (placebo gel) or the test group (0.2% HA gel). The pharmacy department prepared both gels in syringes with equivalent coding.
The applicator tip was gently inserted subgingivally into the periodontal pocket, facilitating the controlled delivery of the gel until the pocket was adequately filled, with the gel visible at the gingival margin, thereby indicating sufficient treatment coverage. Gel administration to T2DM patients was performed by an independent operator (FCH), who remained blinded to the contents of the gel given to each participant. To ensure consistency from the initial assessment to the follow-up phase, participants received thorough guidance on proper oral hygiene and effective brushing techniques. During the 4-week follow-up, all periodontal parameters were re-evaluated, and additional GCF samples were collected. Rigorous protocols for monitoring adverse events were followed, maintaining safety and ethical standards throughout the study.
2.6. Gingival Crevicular Fluid Sampling
Gingival crevicular fluid samples were obtained prior to SRP and again at the 4-week follow-up to assess TNF-α and TGF-β1 levels using enzyme-linked immunosorbent assay (ELISA). Participants were randomly assigned to receive either the 0.2% HA gel or the placebo gel, with a 1:1 allocation ratio, and identical packaging was used to maintain blinding (Fig. 1). The sample sites were dehydrated using an air syringe and secured by placing cotton rolls. Sterile size 25 paper points were subsequently carefully placed into the periodontal pockets for 30 seconds. Following SRP, the gel was applied subgingivally by carefully inserting the applicator tip into the periodontal pocket, ensuring the gel was gradually delivered until visible at the gingival margin, indicating adequate treatment coverage. Paper points exposed to blood or saliva were discarded, while uncontaminated ones were placed into Eppendorf tubes containing PBS solution. Five paper points from each sampling site were obtained and subsequently stored at −20°C until further analysis.
2.7. Measurement of TNF-α and TGF-β1
Samples for TNF-α and TGF-β1 were evaluated using ELISA kits (Bioenzy, China). The concentrations of these cytokines in GCF samples were determined using standard curves. Fifty microliters of the standard solution were added to the standard wells, which were not biotinylated. Each sample (40 µL) was added to the sample wells. Sample replicates were employed to ensure the dependability of the ELISA results. A biotinylated antibody was then introduced into the sample wells, and 50 µL of streptavidin-HRP was added to both the sample and standard wells, ensuring complete and even mixing. The plate was sealed and incubated at 37°C for 60 minutes. Following incubation, the sealant was removed, and the plate was washed five times with a wash buffer. Each wash involved soaking the wells in at least 0.35 mL of wash buffer for 30 to 60 seconds to ensure thorough cleaning. After the washing procedure, 50 µL of substrate solutions A and B were added to each well, and the plate was then sealed and incubated in the dark at 37°C for 10 minutes. Each well received 50 µL of stop solution, resulting in a color change from blue to yellow, and the optical density was read at 450 nm using a microplate reader within 10 minutes. TNF-α and TGF-β1 concentrations were expressed in pg/mL. The protocol followed standard procedures to ensure methodological transparency, including detailed steps for data acquisition, calibration of instruments, sample management, storage procedures, and analysis methods.
2.8. Statistical Analysis
Statistical analysis was conducted using SPSS 26.0 (Statistical Package for the Social Sciences) for Windows. Data normality was assessed using the Shapiro–Wilk test. For normally distributed data, an independent T-test was used to compare the means between two independent groups. In contrast, the Mann-Whitney U-test, a non-parametric alternative, was employed when the normality assumption was not met. Paired T-tests were applied to assess changes in cytokine levels from initial to follow-up for parametric data, while the Wilcoxon signed-rank test was conducted for non-parametric data.
3. RESULTS
The study was conducted between February and June 2023, with a total of 20 subjects. One subject from each group discontinued participation after randomization, leaving 18 patients who completed the clinical trial to the final stage. The participants were evenly distributed, with 9 patients in the placebo gel group and 9 in the 0.2% HA gel group. Both groups received the same treatment, which involved the random application of adjunctive gels following SRP (Fig. 2).
The baseline demographic characteristics of the study participants with T2DM (n=18) are presented in Table 1. The mean age of the participants was 55.83 ± 7.56 years. The gender distribution included 7 males and 11 females.
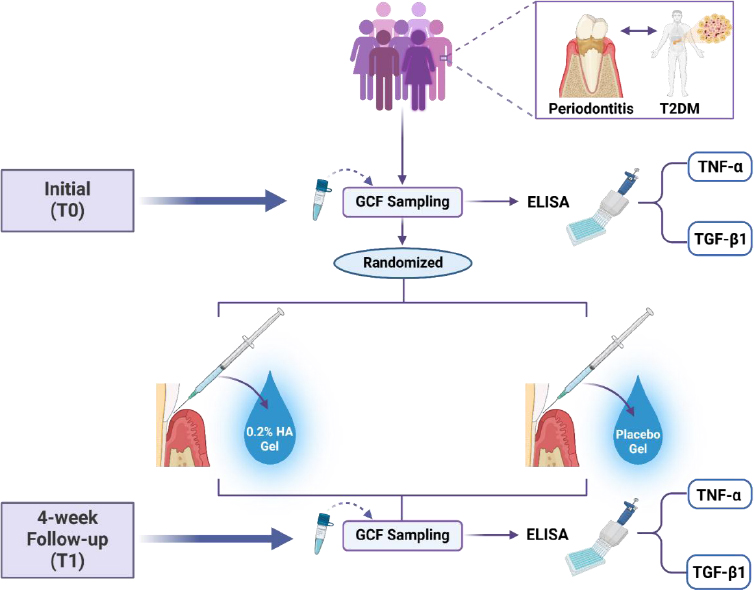
Study design and methodology of hyaluronic acid gel application in periodontitis patients with type 2 diabetes mellitus (created by BioRender.com).
| Baseline Demographic | T2DM (n =18) |
|---|---|
| (mean ± SD) | |
| Age (years) | 55,83 ± 7,563 |
| Gender (M: F) | 7: 11 |
| HbA1c (%) | 7,328 ± 0,690 |
Glycemic control, as indicated by HbA1c levels (7.33 ± 0.69%), confirmed the T2DM diagnosis in all study participants. These baseline characteristics provide essential context for evaluating the impact of 0.2% HA gel on periodontal and biomolecular parameters in T2DM patients.
Biomolecular analysis revealed a significant decrease in TNF-α levels (p<0.05) and an increase in TGF-β1 levels (p<0.05) in both the 0.2% HA gel and placebo gel groups after 4 weeks of follow-up. Although the change in delta values between the initial visit and the 4-week follow-up showed no statistically significant differences (p>0.05) for TNF-α and TGF-β1 levels between the test and control groups in T2DM patients, a clear trend of reduced TNF-α and elevated TGF-β1 was evident (Table 2).
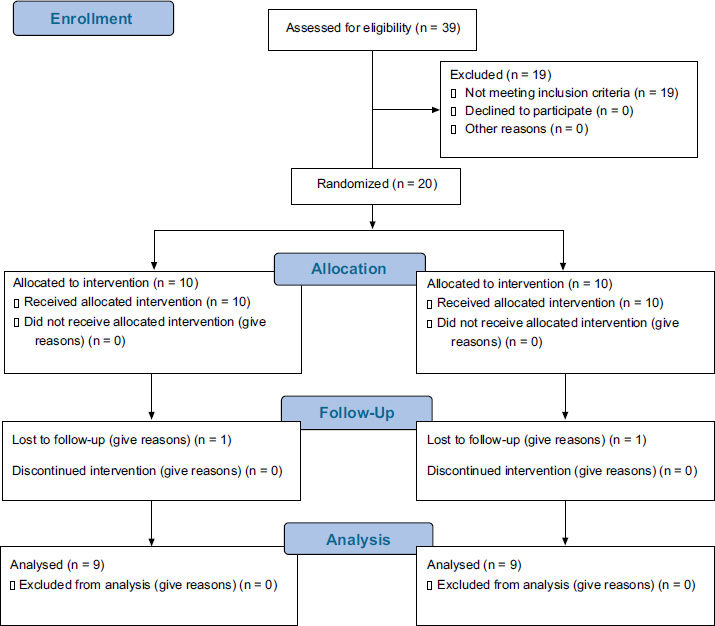
CONSORT flow diagram.
| Level | Groups (n=9) | Mean ± SD (pg/mL) | p* | Δ T0- T1 (pg/mL) | |||
|---|---|---|---|---|---|---|---|
| T0 | p# | T1 | Mean ± SD | p# | |||
|
TNF-α TGF-β1 |
|||||||
| Placebo Gel | 390.23 ± 30.08 | 0.659 [2] | 298.08 ± 63.3 | 0.008 [2]* | 92.14 ± 78.03 | 0.58 [1] | |
| HA Gel 0.2% | 404.41 ± 55.85 | 290.78 ± 58.80 | 0.008 [2]* | 113.62 ± 83.20 | |||
| Placebo Gel | 1373.72 ± 120.00 | 0.710 [2] | 1622.29 ± 76.77 | 0.008 [2]* | -248.57 ± 134.46 | 0.93 [2] | |
| HA Gel 0.2% | 1349.49 ± 150.25 | 1637.08 ± 155.09 | 0.008 [2]* | -287.59 ± 138.96 | |||
4. DISCUSSION
The intricate relationship between periodontitis and T2DM is largely mediated by the heightened inflammatory response and impaired healing processes observed in diabetic patients. In this context, the roles of TNF-α and TGF-β1 are particularly crucial, as these cytokines significantly influence the clinical parameters of periodontal health and disease progression. The cytokine TNF-α plays a pivotal role in the pathogenesis of periodontitis by promoting inflammation and tissue destruction. Elevated levels of TNF-α in periodontal tissues are associated with increased severity of periodontal disease, characterized by deeper periodontal pockets, greater attachment loss, and increased bleeding on probing. In patients with T2DM, the persistent hyperglycemic state further exacerbates the inflammatory response, leading to even higher levels of TNF-α and more severe periodontal outcomes [19].
The findings of our study align with and expand upon previously published research regarding biomolecular and clinical outcomes in T2DM patients undergoing periodontal therapy. Our research demonstrated significant reductions in TNF-α and elevations in TGF-β1 levels in both the test and control groups after 4 weeks, with trends suggesting anti-inflammatory and regenerative effects despite the lack of statistically significant intergroup differences in delta changes. Similarly, other studies have reported decreases in inflammatory markers, such as IL-1β, and increases in anti-inflammatory cytokines, like IL-10, although in some cases, such as the DM HA gel group, the latter did not reach statistical significance. Related research has also shown reductions in microbiological parameters, such as Fusobacterium nucleatum, and significant improvements in clinical parameters, including BOP, PPD, and CAL. These findings collectively highlight the potential of HA-based therapies in reducing inflammation and promoting periodontal health in diabetic and non-diabetic individuals, although the observed differences between groups warrant further exploration [23].
Several studies have demonstrated the potential of HA as a therapeutic agent in the management of periodontitis, particularly through its anti-inflammatory and regenerative effects [5, 23]. As outlined in our study, the use of HA, whether in the form of 0.2% gel or placebo, showed a trend of decreased TNF-α levels and increased TGF-β1 levels, indicating its potential role in modulating inflammation and promoting periodontal tissue regeneration in patients with T2DM.
Beyond the previously mentioned studies, additional research further strengthens the evidence regarding the benefits of HA-based therapy in periodontitis. Hyaluronic acid has been widely studied for its anti-inflammatory and regenerative properties, making it a valuable adjunct in periodontal therapy. HA plays a crucial role in modulating the inflammatory response by regulating cytokine activity, including TNF-α and TGF-β1, which are key mediators in the pathophysiology of periodontitis, particularly in patients with T2DM. Previous studies have demonstrated that HA can promote wound healing by enhancing fibroblast proliferation, extracellular matrix remodeling, and angiogenesis, contributing to tissue regeneration. Bertossi et al. further highlighted the role of HA in regenerative medicine, showing its effectiveness in reducing inflammation and improving tissue repair in skin rejuvenation treatments. These findings support the potential of HA in periodontal applications, where inflammation control and tissue regeneration are crucial for improved clinical outcomes, particularly in patients with compromised healing responses, such as those with T2DM [24].
Other findings also suggest that HA-based therapies play a significant role in periodontal health. A study by Rajan et al. investigated the additional benefits of applying HA gel following standard SRP in patients with chronic periodontitis, contributing to improved periodontal clinical outcomes [25]. Additionally, Zhu et al. found that the potential benefits of HA in reducing biofilm metabolic activity and the modulation of pro-inflammatory cytokine expression, such as IL-8, demonstrated the anti-inflammatory effects of HA, supporting its role in improving periodontal outcomes by reducing inflammation and enhancing tissue repair [26]. Furthermore, Mohammad CA et al. demonstrated that HA gel application significantly reduced pro-inflammatory cytokines, such as IL-1β and TNF-α, highlighting its anti-inflammatory properties. Additionally, clinical improvements in periodontal parameters further support the role of HA in periodontal health. Modulating TNF-α and TGF-β1 levels is crucial for managing periodontitis in T2DM patients, as elevated TNF-α exacerbates inflammation and tissue destruction, while TGF-β1 promotes regeneration. Effective management of periodontitis in patients with T2DM requires therapeutic strategies that not only control infection but also modulate the inflammatory response and promote tissue repair by targeting these critical cytokines [5].
Collectively, these findings provide strong evidence that HA can contribute to periodontal improvement by reducing inflammation and enhancing regenerative processes. This further supports our study’s findings, indicating that the use of 0.2% HA gel may exert anti-inflammatory and regenerative effects in managing periodontitis in T2DM patients. However, the lack of statistically significant differences between groups suggests the need for further research with larger study designs, longer follow-up periods, and varying HA concentrations to optimize its therapeutic benefits in periodontal treatment for patients with T2DM.
CONCLUSION
This study indicates that the application of 0.2% HA as an adjunctive therapy in patients with T2DM and periodontitis significantly modulates key inflammatory mediators. Specifically, 0.2% HA effectively reduces levels of TNF-α, thereby attenuating chronic inflammation while simultaneously increasing levels of TGF-β1, which enhances tissue regeneration. These findings suggest that HA represents a valuable adjunctive treatment option, promoting both anti-inflammatory and regenerative processes in the periodontal healing of T2DM patients.
AUTHORS’ CONTRIBUTIONS
The authors confirm their contribution to the paper as follows: M.M., F.C.H., A.A.D., S.L.M., R.L., and A.W.: Study conception and design; M.M., F.C.H., and A.A.D.: Data collection; M.M., F.C.H., A.A.D., S.L.M., and B.S.: Analysis and interpretation of results; M.M., F.C.H., A.A.D., S.L.M., R.L., A.W., and B.S.: Draft manuscript. All authors reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| GCF | = Gingival Crevicular Fluid |
| T2DM | = Type 2 Diabetes Mellitus |
| DM | = Diabetes Mellitus |
| HA | = Hyaluronic Acid |
| TNF-α | = Tumor Necrosis Factor-alpha |
| TGF-β1 | = Transforming Growth Factor-beta1 |
| LPS | = Like Lipopolysaccharides |
| PPD | = Probing Pocket Depth |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Ethical Commission for Dental Research (KPEKG), Faculty of Dentistry, Universitas Indonesia, Indonesia under approval number 130/Ethical Approval/FKGUI/XII/2022 and protocol reference number 090660722.
HUMAN AND ANIMAL RIGHTS
All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committee and with the 1975 Declaration of Helsinki, as revised in 2013.
CONSENT FOR PUBLICATION
Prior to enrollment, all participants were thoroughly briefed on the study procedures and signed a written informed consent form.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article is available in the Zenodo Repository at https://doi.org/10.5281/zenodo.15594669, reference number ZENODO-15594669.
FUNDING
This research was funded by the Research and Development Directorates of Universitas Indonesia, Indonesia of the 2023 PUTI Q2 grant, NKB-1277/UN2.RST/HKP.05.00/2022.
ACKNOWLEDGEMENTS
Declared none.


