All published articles of this journal are available on ScienceDirect.
Antibacterial Action of Curcumin Nanocrystals on Streptococcus Mutans
Abstract
Aims
The present study aimed to investigate the antibacterial and antibiofilm properties and adhesion inhibitory effect of curcumin nanocrystals compared to bulk curcumin against Streptococcus mutans.
Background
S. mutans is the main aetiological factor in tooth decay. Curcumin has been used in various studies as an antimicrobial agent due to its wide traditional uses and low side effects. Despite having multiple therapeutic effects, its inherent properties, for example, poor aquas solubility, low bioavailability, photodegradation, chemical instability, rapid metabolism, and short half-life, limit its pharmaceutical significance. Recently, newer strategies have been tried to overcome these pharmacological issues and improve curcumin's therapeutic efficacy. The nanoformulation can enhance the effectiveness of curcumin in a wide range of diseases, especially infectious diseases.
Methods
The present study evaluated the Minimum Inhibitory Concentration (MIC), biofilm inhibitory, and adhesion inhibitory actions of curcumin nanocrystals compared to bulk curcumin against S. mutans.
Results
The results showed that curcumin nanocrystals were more effective than curcumin against S. mutans. The MICs spectrum of bulk curcumin and curcumin nanocrystals was 256-512 µg/mL and 125-256 µg/mL, respectively. Both bulk curcumin and curcumin nanocrystals inhibited the formation of S. mutans biofilm (p<0.05), and bacterial adhesion was significantly decreased compared to bulk curcumin and control (p<0.05).
Conclusion
These findings showed that using curcumin nanocrystals in mouthwashes and toothpaste can be useful in preventing dental plaque. Further research is required to determine the stability of curcumin nanocrystals in formulation with other dental product ingredients and to assess their safety and efficacy in human clinical trials.
1. INTRODUCTION
In dental caries, Streptococcus mutans is the main aetiological factor [1, 2]. This bacterium can adhere to solid surfaces, colonize the oral cavity, and create biofilms [3]. A dental bacterial biofilm is a bacterial mass formed in a self-generating extracellular matrix. Bacteria in biofilm formation have a completely different phenotype from the planktonic form [4]. The bacteria in the biofilm of teeth are much more resistant to adverse growth situations such as hydrodynamic shear forces and biocides [5, 6]. Through Glycosyltransferases (Gtfs), S. mutans produces Extracellular Polysaccharide (EPS), which forms the biofilm matrix [7]. EPS intermediates permanent adhesion between bacteria to form a biofilm with high cell density that increases the resistance of bacteria to antibiotics [8].
Today, natural substances are increasingly considered as alternative or adjunctive anti-caries treatments. Plant-derived molecules, in particular, are often superior to synthetic derivatives due to their natural evolution and reduced risk of resistance [9, 10].
Curcumin, the main component of turmeric or Curcuma longa L., is considered a potentially satisfying antimicrobial agent [11, 12]. Curcumin can prevent the growth of bacteria such as Mycobacterium tuberculosis, Staphylococcus aureus, Trichophyton gypseum, and Salmonella paratyphi [11, 13]. Recent studies displayed that curcumin is effective against drug-resistant bacteria [14]. Curcumin is effective against dental disease-related oral bacteria [15]. Song et al. revealed that curcumin can prevent the adhesion of S. mutansvia its effect on fibronectin and collagen [14]. The study of Hu et al. showed that curcumin is an inhibitor of S. mutans sortase A and has capable anti-caries properties based on the anti-adhesion mechanism [16]. Furthermore, the planktonic form of S. mutans can be photoinactivation via blue light-activated curcumin, but its effect on the biofilm form is weak [17]. Therefore, these investigations suggested the potential usage of curcumin as a natural antibacterial agent and emphasized the necessity of studying how curcumin acts on oral bacteria.
Nanotechnology has wide application in all research fields [18-21]. In biomedicine, the development of drug delivery through nanoparticle systems has led to a significant increase in technological innovations [22, 23]. Nanomedicine has opened new opportunities for the modern medicine. Increasing the bioavailability of active substances is one of the advantages of pharmaceutical nanoformulations [24, 25].
Nanotechnology offers innovative methods for the prevention, treatment, and diagnosis of dental diseases [26-28]. This novel field, known as nano dentistry, involves the usage of nanomaterials, nanorobots, and nanotechnology-based methods to improve dental care [29-31].
The present study was conducted to investigate the antibacterial effect and biofilm inhibition of curcumin nanocrystals against S. mutans. In addition, the curcumin nanocrystals' effect on S. mutans adhesion to surfaces was investigated.
2. MATERIAL AND METHODS
2.1. Prepare Curcumin Nanocrystals
Curcumin nanocrystals were produced in our previous study through evaporative precipitation along with spray-drying methods [32]. Curcumin was first dissolved in ethanol and then hexane was added rapidly to it, and the ratio of ethanol to hexane was 1/30 by volume. Curcumin nanocrystals were obtained by evaporating the solvent through a rotary evaporator. The spray drying was performed with inlet and outlet temperatures of 150°C and 80°C, respectively, and at a liquid feed rate of 1.5 mL/min. The prepared nanocrystals had an average particle size of 95 nm and had a small spherical and uniform shape [32].
2.2. The Antibacterial Activity
In the present study, S. mutans was isolated from Tabriz, Iran, in 2020. The Minimum Inhibitory Concentration (MIC) was determined to evaluate the antibacterial effect of curcumin nanocrystals and bulk curcumin via the broth microdilution method in Cation-Adjusted MuellerHinton Broth (CAMHB) [33]. MIC was considered bacterial growth inhibition by the lowest concentration of the compound, which was identified by the naked eye.
2.3. Biofilm Inhibitory Effect
The Minimum Biofilm Inhibitory Concentration (MBIC) was used to determine the anti-biofilm effect of curcumin nanocrystals and bulk curcumin. Firstly, bacterial suspension (0.5 McFarland) was prepared, and 100 µL was added to 100 µL of TSB in microplate wells. For stimulation of the bacterial biofilm generation, 0.25% glucose was added to TSB and incubated at 35°C for 24 hours. Then, the serial concentrations of bulk curcumin and curcumin nanocrystals were added, and the microplate was incubated at 35 °C for 20 hours. Then, the well's content was removed, rinsed with sterile water, and shaken. Finally, the OD of the wells was evaluated before and after incubation at 35 °C for 6 hours at a wavelength of 650 nm.
The MBIC was evaluated as the lowest compound level which lid in an OD 650 change at or less than 10% of the mean OD of positive control wells.
2.4. Bacterial Attachment
Sub-inhibitory concentration (0.5 MIC) of curcumin nanocrystals and bulk curcumin were used to evaluate the effect of curcumin nanocrystals and bulk curcumin on the development of biofilm. TSB medium (1:20) with and without curcumin nanocrystals and bulk curcumin were utilized for the dilute bacteria that grew overnight. Then, the suspension was poured into the wells, and the microplate was incubated for 24 hours at 35°C. After removing the contents of the wells, the biofilms were rinsed with water. Methanol was used for the fixation of biofilms for 5 minutes. After drying, crystal violet solution was used, staining for 15 minutes. After washing, the acetic acid (33%) was used to dissolve biofilms. Lastly, the wells’ OD was read at wavelength 492 nm through a plate reader. TSB without bacteria was considered a negative control.
3. RESULTS
Curcumin displayed a significant antibacterial effect on S. mutans. The Curcumin nanocrystals inhibited S. mutans significantly more than bulk curcumin (p<0.05) (Fig. 1). The MICs spectrum of curcumin and curcumin nanocrystals was 256-512 µg/mL and 125-256 µg/mL, respectively.
In this study, both bulk curcumin and curcumin nanocrystals inhibited the formation of S. mutans biofilm (p<0.05) (Fig. 2).
The adhesion of bacterial cells to surfaces was studied to evaluate the effect of curcumin nanocrystals at sub-MIC levels (0.5 MIC). After 12 hours of incubation in sub-MIC levels of curcumin nanocrystals, bacterial adhesion was significantly reduced compared to bulk curcumin and control (p<0.05) (grown of bacteria without curcumin nanocrystals) (Fig. 3).
4. DISCUSSION
It has been shown that nanoparticles may affect bacteria because of two probable mechanisms: (a) nanoparticles may combine with the surface of bacteria and enhance the permeability of bacterial cells, and (b) they may also be absorbed and penetrate the bacterial cell wall and act as a depot for the release of antibacterial drugs [34]. Depending on the size of nanoparticles and bacterial type, nanoparticles apply their antibacterial properties on bacteria with different mechanisms, and the mechanisms are different from those reported for the common antibiotics. For this purpose, using nanoparticles with antimicrobial effects is a way to overcome the microbial resistance of antibiotics [35]. Membrane-nanoparticle interaction creates local pores in the membrane, allowing nanoparticles to enter the bacteria and interact with DNA and intracellular proteins (especially sulfur-rich protein). They damage the bacteria to a great extent. Another possible antibacterial mechanism includes the attachment of nanoparticles to the bacterial membrane and their ongoing entrance into the bacterial cytoplasm and interruption of its function. Some pharmaceutical nanoformulations can also combine with the cell walls of bacteria and inject their antibacterial substances into the bacteria [36, 35].
Nanoformulations of curcumin have improved its antimicrobial effects against different microbes, such as S. aureus, Escherichia coli, Bacillus subtilis, Aspergillus, Saccharomyces cerevisiae, Yersinia enterocolitica, and B. cereus [37, 38].
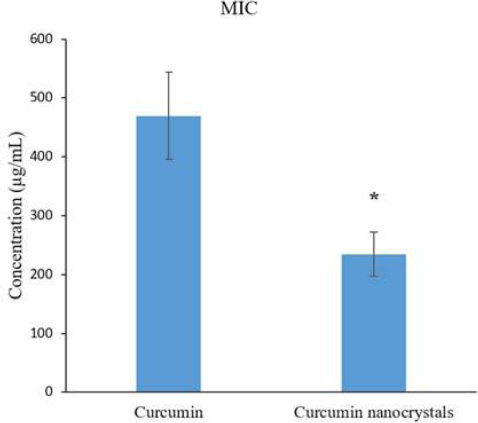
The inhibitory effects of curcumin nanocrystals on S. mutans compared with curcumin.
Note: (*) indicates a statistically significant (p≤0.05).
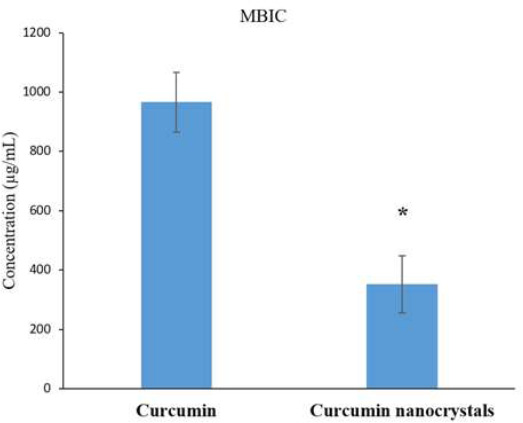
The biofilm inhibitory action of curcumin nanocrystals on S. mutans compared with bulk curcumin.
Note: An asterisk (*) shows a statistically significant difference (p≤0.05).
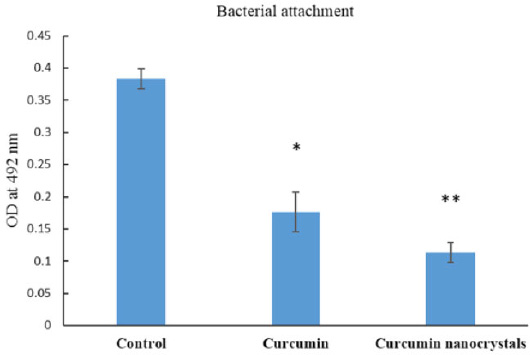
Effect of bulk curcumin and curcumin nanocrystals on the adhesion of S. mutans to plastic surfaces. Bacteria growing without any treatment was considered control.
Note: (*) compared with control, (**) compared with bulk curcumin (p≤0.05).
Negahdari et al. prepared and studied the antibacterial effect of nanocrystals of curcumin against S. aureus, E. coli, and Enterococcus faecalis inside the implant fixture. Their results displayed that bacterial Colony-Forming Unit (CFU) in exposure to curcumin nanocrystals significantly decreased (p < 0.01) by the incubation time [32]. Maleki Dizaj et al. investigated the antimicrobial effects of nanocrystals of curcumin against Porphyromonas gingivalis, which was isolated from patients with dental implant failure. Curcumin nanocrystals had MBC of 12.5 μg/mL and MIC of 6.25 μg/mL against P. gingivalis [39].
Antibiotics are generally effective against bacterial planktonic form but may not be efficient against bacterial biofilm form [40]. Suitable drug delivery systems for antimicrobial agents can be useful in infection treatments caused by the biofilm form of bacteria.
Biofilms removal is difficult and they are resistant to most antimicrobial agents. Sometimes, the elimination of infected tissue or apparatus is required, which is undesirable and sometimes impossible [41]. Therefore, biofilm elimination may need the usage of new compounds or formulations that increase the sensitivity of biofilms to antibacterial agents [42]. Nanotechnology offers new and innovative solutions for antibacterial drug delivery and biofilm disruption and offers new possibilities for preventive/therapeutic approaches for biofilm-associated oral diseases [43-45]. In this study, the results showed both bulk curcumin and curcumin nanocrystals inhibited the formation of S. mutans biofilm (p<0.05). However, more in vitro and in vivo studies are suggested for further investigation of the anti-biofilm effect of the curcumin nanocrystals.
Adhesion is a critical factor in the colonization of bacteria on various surfaces. The results of this study displayed that curcumin nanocrystals decreased bacterial adhesion at sub-MIC levels in S. mutans (p<0.05).
CONCLUSION
In the present study, results showed that curcumin nanocrystals have more antibacterial action compared to bulk curcumin. Furthermore, bulk curcumin and curcumin nanocrystals display biofilm inhibitory action. Curcumin nanocrystals decreased the bacterial attachment at sub-MIC levels.
This study demonstrated the antimicrobial effects of curcumin nanocrystals in vitro. However, clinical translation may be challenged by issues such as nanoparticle toxicity, stability in oral environments, and scalability of production processes. Further studies are needed to ensure biocompatibility and safe use in humans. Therefore, the properties of nanoparticles, toxicity, biocompatibility, and other factors should be considered during clinical trials to ensure its performance as an antibacterial and antibiofilm agent with minimal tolerable side effects.
Furthermore, these results may be useful for the application of curcumin nanocrystals in oral and dental products such as mouthwashes and toothpaste to prevent dental plaque. Further research is required to determine the stability of curcumin nanocrystals in formulation with other dental product ingredients and to assess their safety and efficacy in human clinical trials. In vivo and clinical surveys on the biological actions of curcumin nanocrystals are essential for mentioned applications.
AUTHORS' CONTRIBUTION
The authors confirm their contribution to the paper: S.S. and S.M.D.: Study conception and design; M.Y.M.: Data collection; M.Y.M., S.S. and S.M.D.: Analysis and interpretation of results; M.Y.M., M.Y., Y.R., and M.N.: Draft manuscript. All authors reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| CAMHB | = Cation-Adjusted Mueller–Hinton Broth |
| CFU | = Colony-Forming Unit |
| EPS | = Extracellular Polysaccharide |
| Gtfs | = Glycosyltransferases |
| MBIC | = Minimum Biofilm Inhibitory Concentration |
| MIC | = Minimum Inhibitory Concentration |
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article will be available from the corresponding author [S.S] upon reasonable request.
FUNDING
This article was prepared with the financial support of Tabriz University of Medical Sciences, Iran from the thesis (No. 73117), and the authors are grateful for their financial support.
ACKNOWLEDGEMENTS
Declared none.


